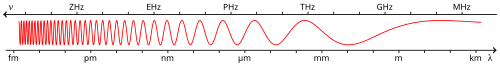
Electromagnetic spectrum

γ = Gamma rays
HX = Hard X-rays
SX = Soft X-Rays
EUV = Extreme ultraviolet
NUV = Near ultraviolet
Visible light
NIR = Near infrared
MIR = Mid infrared
FIR = Far infrared
Radio waves
EHF = Extremely high frequency (Microwaves)
SHF = Super high frequency (Microwaves)
UHF = Ultra high frequency
VHF = Very high frequency
HF = High frequency
MF = Medium frequency
LF = Low frequency
VLF = Very low frequency
VF = Voice frequency
ELF = Extremely low frequency
The electromagnetic (EM) spectrum is the range of all possible electromagnetic radiation. The "electromagnetic spectrum" (usually just spectrum) of an object is the frequency range of electromagnetic radiation with wavelengths from thousands of kilometers down to fractions of the size of an atom. It is commonly said[citation needed] that EM waves beyond these limits are uncommon, although this is not actually true. The short wavelength limit is likely to be the Planck length, and the long wavelength limit is the size of the universe itself (see physical cosmology), though in principle the spectrum is infinite.
Electromagnetic energy at a particular wavelength λ (in vacuum) has an associated frequency f and photon energy E. Thus, the electromagnetic spectrum may be expressed equally well in terms of any of these three quantities. They are related according to the equations:
- wave speed (c) = frequency x wavelength
or
and
or
where:
- c is the speed of light, 299,792,458 m/s (exact).
- h is Planck's constant, .
So, high-frequency electromagnetic waves have a short wavelength and high energy; low-frequency waves have a long wavelength and low energy.
When light waves (and other electromagnetic waves) enter a medium, their wavelength is reduced. Wavelengths of electromagnetic radiation, no matter what medium they are travelling through, are usually quoted in terms of the vacuum wavelength, although this is not always explicitly stated.
Spectra of objects

Nearly all observable objects in the universe emit, reflect or transmit some light. (One hypothetical exception may be dark matter, which, along with Dark energy make up 96% of the universe's total mass.) The distribution of this light along the electromagnetic spectrum (called the spectrum of the object) is determined by the object's composition. Several types of spectra can be distinguished depending upon the nature of the radiation coming from an object:
- If the spectrum is composed primarily of thermal radiation emitted by the object itself, an emission spectrum occurs.
- Some bodies emit light more or less according to the blackbody spectrum.
- If the spectrum is composed of background light, parts of which the object transmits and parts of which it absorbs, an absorption spectrum occurs.
Electromagnetic spectroscopy is the branch of physics that deals with the characterization of matter by its spectra.

A Halogen incandescent lamp, has a light spectra as shown in the accompanying figure. The figure shows just the infrared end of the whole spectra, which is limited by the resolution of the spectrum analyzer from 600 nm to 1500 nm, in the optical window, typical to fiber optic communication systems.
Wave characteristics
External links
- U.S. Frequency Allocation Chart — Covering the range 3 kHz to 300 GHz (from Department of Commerce)
- Canadian Table of Frequency Allocations (from Industry Canada)
- UK frequency allocation table (from Ofcom, which inherited the Radiocommunications Agency's duties, pdf format)
- The Science of Spectroscopy - supported by NASA, includes OpenSpectrum, a Wiki-based learning tool for spectroscopy that anyone can edit