Folding@home
| The Folding@home icon. | |
| Original author(s) | Vijay Pande |
|---|---|
| Developer(s) | Stanford University / Pande Group |
| Stable release | 5.04 (Windows CLI), 5.03 (Windows GUI), 5.04 (Linux and BSD), 5.02 (Mac OS X-PPC), 1.31 (PlayStation 3) |
| Preview release | 6.10beta2 (Windows CLI and GUI), 6.01beta2 (Mac OS X-PPC), 6.00beta2 (Linux-SMP & Uniprocessor), 6.10beta2 (GPU GUI and CLI), 5.91beta6 (Windows-SMP), 6.10beta2 (Mac OS X-x86-SMP) / 2008-03-05 |
| Platform | Cross-platform |
| Type | Distributed computing |
| License | Proprietary [1] |
| Website | folding.stanford.edu |
Folding@home (also known as FAH or F@H) is a distributed computing (DC) project designed to perform computationally intensive simulations of protein folding and other molecular dynamics (MD). It was launched on October 1, 2000, and is currently managed by the Pande Group, within Stanford University's chemistry department, under the supervision of Professor Vijay Pande. Folding@home is the most powerful distributed computing cluster in the world, according to Guinness,[1] and one of the world's largest distributed computing projects.[2] The goal of the project is "to understand protein folding, misfolding, and related diseases."[3]
Purpose
Accurate simulations of protein folding and misfolding enable the scientific community to better understand the development of many diseases, including sickle-cell disease (drepanocytosis), Alzheimer's disease, Parkinson's disease, BSE (mad cow disease), cancer, Huntington's disease, cystic fibrosis, osteogenesis imperfecta, alpha 1-antitrypsin deficiency, and other aggregation-related diseases. [2] More fundamentally, understanding the process of protein folding — how biological molecules assemble themselves into a functional state — is one of the outstanding problems of molecular biology. So far, the Folding@home project has successfully simulated folding in the 5-10 microsecond range — a time scale thousands of times longer than it was previously thought possible to model.[4] The Pande Group goal is to refine and improve the MD and Folding@home DC methods to the level where it will become an essential tool for the MD research. [5] For that goal they collaborate with various scientific institutions. [6] As of December 13, 2007, fifty-four scientific research papers have been published using the project's work.[7] A University of Illinois at Urbana-Champaign report dated October 22, 2002 states that Folding@home distributed simulations of protein folding are demonstrably accurate.[8]
How it works

Folding@home does not rely on powerful supercomputers for its data processing; instead, the primary contributors to the Folding@home project are many hundreds of thousands of personal computer users who have installed a small client program. The client will, at the user's choice, run in the background, utilizing otherwise unused CPU power, or run as a screensaver only while the user is away. In most modern personal computers, the CPU is rarely used to its full capacity at all times; the Folding@home client takes advantage of this unused processing power.
The Folding@home client periodically connects to a server to retrieve "work units," which are packets of data upon which to perform calculations. Each completed work unit is then sent back to the server. As data integrity is a major concern for all distributed computing projects, all work units are validated through the use of a 2048 bit digital signature.
Contributors to Folding@home may have user names used to keep track of their contributions. Each user may be running the client on one or more CPUs; for example, a user with two computers could run the client on both of them. Users may also contribute under one or more team names; many different users may join together to form a team. Contributors are assigned a score indicating the number and difficulty of completed work units. Rankings and other statistics are posted to the Folding@home website.
Analysis Software
The Folding@home client utilizes modified versions of four molecular simulation programs for calculation: TINKER, GROMACS, AMBER, and CPMD.[9] Where possible, optimizations are used to speed the process of calculation. There are many variations on these base simulation programs, each of which is given an arbitrary identifier (Core xx):[10]
- TINKER
- Tinker core (Core 65)
- Currently inactive, as the GBGromacs core (Core 7a) performs the same tasks much faster.
- No optimizations
- Tinker core (Core 65)
- GROMACS (all variants of this core use SSE, 3DNow+ or AltiVec optimizations, where available, unless otherwise specified)
- Gromacs (Core 78)
- Double Gromacs (Core 79)
- Double Precision, uses SSE2 only.
- Double Gromacs B (Core 7b)
- Nominally an update of Double Gromacs, but is actually based on the SMP/GPU codebases (and is therefore a completely new core). As a result, both are still in use.
- Double precision, uses SSE2 only.
- GBGromacs (Core 7a)
- Gromacs with the Generalized Born implicit solvent model
- Gromacs SREM (Core 80)
- Gromacs Serial Replica Exchange Method
- The Gromacs Serial Replica Exchange Method core, also known as GroST (Gromacs Serial replica exchange with Temperatures), uses Replica Exchange Method (also known as REMD or Replica Exchange Molecular Dynamics) in its simulations.
- GroSimT (Core 81)
- Gromacs with Simulated Tempering
- Gromacs 33 (Core a0)
- uses the newer Gromacs 3.3 codebase
- Gro-SMP v1.74 (Core a1)
- Gro-SMP v1.90 (Core a2)
- Gro-GPU (Core 10)
- Graphics Processing Unit variant
- GPUs do not have optimizations; they are powerful enough to do the calculations simply using brute force
- Gro-PS3 (Does not have a known ID number, but also called SCEARD core)
- PlayStation 3 variant
- No optimizations, see GPU variant
- AMBER
- PMD core (Core 82)[10]
- No optimizations.
- PMD core (Core 82)[10]
- CPMD
Possible future additions:
Participation
Shortly after breaking the 200,000 active CPU count on September 20, 2005, the Folding@home project celebrated its fifth anniversary on October 1, 2005.
As of January 5, 2008, the Folding@home project has received computational results from over 2.7 million devices[2] over the course of its time.
Interest and participation in the project has grown steadily since its launch. The number of active devices participating in the project increased substantially after receiving much publicity during the launch of their High Performance clients for both ATi Graphics Cards and the PlayStation 3.
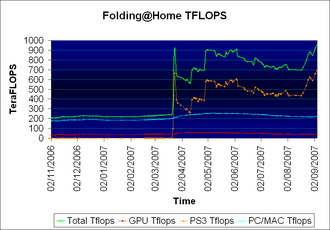
As of November 3, 2007 the peak speed of the project overall has reached over 1.5 PFLOPS.[2]
PetaFLOP Milestone
On September 16, 2007, the Folding@home project officially attained a performance level higher than one petaFLOPS, becoming the first computing system of any kind to do so, although it had briefly peaked above one petaFLOPS in March 2007.[12][13]. In comparison, the fastest supercomputer in the world (as of November 2007, IBM's Blue Gene/L supercomputer) peaks at 478.2 teraFLOPS (1000 teraFLOPS=1 petaFLOP). The Folding@home supercomputer currently operates at above 1 petaFLOPs at all times, with a large majority of the performance coming from PlayStation 3 clients.[2]
Google & Folding@home
There used to be cooperation between Folding@home and Google Labs in the form of Google Toolbar. Google Compute supported Folding@home during its early stage — when Folding@home had ~10,000 active CPUs. At that time, a boost of 20,000 machines was very significant. Today the project has a large number of active CPUs and the number of new clients joining Google Compute was very low (most people opted for the Folding@home client instead), so it was discontinued. The Google Compute clients also had certain limits: they could only run the TINKER core and had limited naming and team options. Folding@home is no longer supported on Google Toolbar, and even the old Google Toolbar client will not work.[14]
Genome@home
Folding@home absorbed the Genome@home project in March 2004. The work which was started by the Genome@home project has since been completed using the Folding@home network (the "Deadlineless" Work Units), and no new work is being distributed by this project. All donators were encouraged to download the Folding@home client (the F@H 4.xx client had a Genome@home option), and once the Genome@home work was complete these clients were asked to donate their processing power to the Folding@home project instead.
Results
These peer-reviewed papers (in chronological order) all use research from the Folding@home project.[7]
2000-2001
- Screen savers of the world, Unite!
Michael R. Shirts and Vijay Pande, Science (2000)
- Mathematical Foundations of ensemble dynamics.
Michael R. Shirts and Vijay Pande, Physical Review Letters (2001)
- b-Hairpin Folding Simulations in Atomistic Detail Using an Implicit Solvent Model.
Bojan Zagrovic, Eric J. Sorin, and Vijay Pande, Journal of Molecular Biology (2001)
2002
- Atomistic protein folding simulations on the submillisecond timescale using worldwide distributed computing.
Vijay Pande, et al. Peter Kollman Memorial Issue, Biopolymers (2002)
- Folding@home and Genome@home: Using distributed computing to tackle previously intractable problems in computational biology.
Stefan M. Larson, Christopher D. Snow, Michael R. Shirts, and Vijay S. Pande. To appear in Computational Genomics, Richard Grant, editor, Horizon Press (2002)
- Simulation of Folding of a Small Alpha-helical Protein in Atomistic Detail using Worldwidedistributed Computing.
Bojan Zagrovic, Christopher D. Snow, Michael R. Shirts, and Vijay S. Pande. Journal of Molecular Biology (2002)
- Native-like Mean Structure in the Unfolded Ensemble of Small Proteins.
Bojan Zagrovic, Christopher D. Snow, Siraj Khaliq, Michael R. Shirts, and Vijay S. Pande. Journal of Molecular Biology (2002)
- Absolute comparison of simulated and experimental protein-folding dynamics.
Christopher D. Snow, Houbi Ngyen, Vijay S. Pande, and Martin Gruebele. Nature (2002)
- The Trp Cage: Folding Kinetics and Unfolded State Topology via Molecular Dynamics Simulations.
Christopher D. Snow, Bojan Zagrovic, and Vijay S. Pande. Journal of the American Chemical Society (2002)
2003
- Multiplexed-Replica Exchange Molecular Dynamics Method for Protein Folding Simulation.
Young Min Rhee & Vijay S. Pande. Biophysical Journal (2003)
- Insights Into Nucleic Acid Conformational Dynamics from Massively Parallel Stochastic Simulations.
Eric J. Sorin, Young Min Rhee, Bradley J. Nakatani & Vijay S. Pande. Biophysical Journal (2003)
- Solvent Viscosity Dependence of the Folding Rate of a Small Protein: Distributed Computing Study.
Bojan Zagrovic and Vijay S. Pande. Journal of Computational Chemistry (2003)
- Extremely precise free energy calculations of amino acid side chain analogs: Comparison of common molecular mechanics force fields for proteins.
Michael R. Shirts, Jed W. Pitera, William C. Swope, and Vijay S. Pande. Journal of Chemical Physics (2003)
- Equilibrium Free Energies from Nonequilibrium Measurements Using Maximum-Likelihood Methods.
Michael R. Shirts, Eric Bair, Giles Hooker, and Vijay S Pande. Physical Review Letters (2003)
- Structural correspondence between the alpha-helix and the random-flight chain resolves how unfolded proteins can have native-like properties.
Bojan Zagrovic & Vijay S Pande. Nature Structural Biology (2003)
2004
- Does Native State Topology Determine the RNA Folding Mechanism?
Eric J. Sorin, Bradley J. Nakatani, Young Min Rhee, Guha Jayachandran, V Vishal, & Vijay S Pande. Journal of Molecular Biology (2004)
- Trp zipper folding kinetics by molecular dynamics and temperature-jump spectroscopy.
Christopher D. Snow, Linlin Qiu, Deguo Du, Feng Gai, Stephen J. Hagen, & Vijay S Pande. Proceedings of the National Academy of Sciences, USA (2004)
- Simulations of the role of water in the protein-folding mechanism.
Young Min Rhee, Eric J. Sorin, Guha Jayachandran, Erik Lindahl, & Vijay S Pande. Proceedings of the National Academy of Sciences, USA(2004)
- Using path sampling to build better Markovian state models: Predicting the folding rate and mechanism of a tryptophan zipper beta hairpin.
Nina Singhal, Christopher D. Snow, and Vijay S. Pande. Journal of Chemical Physics (2004)
2005
- Dimerization of the p53 Oligomerization Domain: Identification of a Folding Nucleus by Molecular Dynamics Simulations.
Lillian T. Chong, Christopher D. Snow, Young Min Rhee, and Vijay S. Pande. Journal of Molecular Biology (2005)
- Does Water Play a Structural Role in the Folding of Small Nucleic Acids?
Eric J. Sorin, Young Min Rhee, and Vijay S. Pande. Biophysical Journal (2005)
- Exploring the Helix-Coil Transition via All-atom Equilibrium Ensemble Simulations.
Eric J. Sorin and Vijay S. Pande. Biophysical Journal (2005)
- Empirical Force-Field Assessment: The Interplay Between Backbone Torsions and Noncovalent Term Scaling.
Eric J. Sorin and Vijay S. Pande. Journal of Computational Chemistry (2005)
- How well can simulation predict protein folding kinetics and thermodynamics?
Christopher D. Snow, Eric J. Sorin, Young Min Rhee, and Vijay S. Pande. Annual Review of Biophysics & Biomolecular Structure (2005)
- Unusual compactness of a polyproline type II structure.
Bojan Zagrovic, Jan Lipfert, Eric J. Sorin, Ian S. Millett, Wilfred F. van Gunsteren, Sebastian Doniach & Vijay S. Pande. Proceedings of the National Academy of Sciences, USA(2005)
- Foldamer dynamics expressed via Markov state models. II. State space decomposition.
Sidney Elmer, Sanghyun Park, & Vijay S. Pande. Journal of Chemical Physics (2005)
- Foldamer dynamics expressed via Markov state models. I. Explicit solvent molecular-dynamics simulations in acetonitrile, chloroform, methanol, and water.
Sidney Elmer, Sanghyun Park, & Vijay S. Pande. Journal of Chemical Physics (2005)
- Solvation free energies of amino acid side chain analogs for common molecular mechanics water models.
Michael R. Shirts & Vijay S. Pande. Journal of Chemical Physics (2005)
- Comparison of efficiency and bias of free energies computed by exponential averaging, the Bennett acceptance ratio, and thermodynamic integration.
Michael R. Shirts & Vijay S. Pande. Journal of Chemical Physics (2005)
- A New Set of Molecular Mechanics Parameters for Hydroxyproline and Its Use in Molecular Dynamics Simulations of Collagen-Like Peptides.
Sanghyun Park, Randall J. Radmer, Teri E. Klein, and Vijay S. Pande. Journal of Computational Chemistry (2005)
- Direct calculation of the binding free energies of FKBP ligands using the Fujitsu BioServer massively parallel computer.
Hideaki Fujutani, Yoshiaki Tanida, Masakatsu Ito, Guha Jayachandran, Christopher D. Snow, Michael R. Shirts, Eric J. Sorin, and Vijay S. Pande. Journal of Chemical Physics (2005)
- Error Analysis in Markovian State Models for protein folding.
Nina Singhal and Vijay S. Pande. Journal of Chemical Physics (2005)
- How large is alpha-helix in solution? Studies of the radii of gyration of helical peptides by SAXS and MD.
Bojan Zagrovic, Guha Jayachandran, Ian S. Millett, Sebastian Doniach and Vijay S. Pande. Journal of Molecular Biology (2005)
- Can conformational change be described by only a few normal modes?
Paula Petrone and Vijay S. Pande. Biophysical Journal (2005)
2006
- The solvation interface is a determining factor in peptide conformational preferences.
Eric J. Sorin, Young Min Rhee, Michael R. Shirts, and Vijay S. Pande. Journal of Molecular Biology (2006)
- Nanotube confinement denatures protein helices.
Eric J. Sorin and Vijay S. Pande. JACS (2006)
- On the role of chemical detail in simulating protein folding kinetics.
Young Min Rhee and Vijay S. Pande. Journal of Chemical Physics (2006)
- Validation of Markov state models using Shannon's entropy.
S. Park and V. S. Pande. Journal of Chemical Physics (2006)
- A novel approach for computational alanine scanning: application to the p53 oligomerization domain.
L.T. Chong, W. C. Swope, J. W. Pitera, and V. S. Pande. Journal of Molecular Biology (2006)
- Electric Fields at the Active Site of an Enzyme: Direct Comparison of Experiment with Theory.
Ian T. Suydam, Christopher D. Snow, Vijay S. Pande, Steven G. Boxer. Science (2006)
- Ensemble molecular dynamics yields submillisecond kinetics and intermediates of membrane fusion.
P. Kasson, N. Kelley, N. Singhal, M. Vrjlic, A. Brunger, and V. S. Pande. Proceedings of the National Academy of Sciences, USA (2006)
- Folding Simulations of the Villin Headpiece in All-Atom Detail.
Guha Jayachandran, V. Vishal, and V. S. Pande. Journal of Chemical Physics (2006)
- Parallelized Over Parts Computation of Absolute Binding Free Energy with Docking and Molecular Dynamics.
Guha Jayachandran, M. R. Shirts, S. Park, and V. S. Pande. Journal of Chemical Physics (2006)
- Kinetic Definition of Protein Folding Transition State Ensembles and Reaction Coordinates.
C. Snow and V. S. Pande. Biophysical Journal (2006)
- Local structure formation in simulations of two small proteins.
Guha Jayachandran, V. Vishal, Angel E. Garcıa and V. S. Pande. Journal of Structural Biology (2006)
- A Bayesian Update Method for Adaptive Weighted Sampling.
S. Park and V. S. Pande. Physical Review (2006)
- Predicting structure and dynamics of loosely-ordered protein complexes: influenza hemagglutinin fusion peptide.
P. Kasson and V. S. Pande. PSB (2006)
2007
- Storage@home: Petascale Distributed Storage
Adam L Beberg and Vijay S. Pande. IPDPS (2007)
- Automatic State Decomposition Algorithm.
J. Chodera, N. Singhal, V. S. Pande, K. Dill, and W. Swope. Journal of Chemical Physics (2007)
- Protein folding under confinement: a role for solvent.
D. Lucent, V. Vishal, V. S. Pande. Proceedings of the National Academy of Sciences, USA (2007)
- Persistent voids: a new structural metric for membrane fusion.
P. M. Kasson, A. Zomorodian, S. Park, N. Singhal, L. J. Guibas, and V. S. Pande. Bioinformatics (2007)
- Control of Membrane Fusion Mechanism by Lipid Composition: Predictions from Ensemble Molecular Dynamics.
P. M. Kasson and V. S. Pande. PLoS Computational Biology (2007)
- Heterogeneity Even at the Speed Limit of Folding: Large-scale Molecular Dynamics Study of a Fast-folding Variant of the Villin Headpiece
D. Ensign, P. M. Kasson, and V. S. Pande. Journal of Molecular Biology (2007)
- Calculation of the distribution of eigenvalues and eigenvectors in Markovian state models for molecular dynamics.
High performance platforms

Graphical processing units
As of October 2, 2006, the Folding@home GPU client has been released into the public as a beta test. After 9 days of processing from the Beta client the Folding@home project had received 31 teraFLOPs of computational performance from just 450 X1900 GPUs, averaging at over 70x the performance of current CPU submissions.[2] The next FAH GPU client will support 2xxx/3xxx series of ATI GPUs, there are no FAH GPU clients for Nvidia GPUs.[15]
PlayStation 3
Stanford announced in August 2006 that a folding client was available to run on the Sony PlayStation 3.[16] The intent was that gamers would be able to contribute to the project by merely "contributing electricity," leaving their PlayStation 3 consoles running the client while not playing games. PS3 firmware version 1.6 (released on Thursday, March 22, 2007) allows for Folding@home software, a 50 MB download, to be used on the PS3.[2] A peak output of the project at 990 teraFLOPS was achieved on 25 March, 2007, at which time the number of FLOPS from each PS3 as reported by Stanford fell, reducing the overall speed rating of those machines by 50%. This had the effect of bumping down the overall project speed to the mid 700 range and increasing the number of active PS3s required to achieve a petaFLOPS level to around 60,000. Lately, the console accounts for about 60% of all teraFLOPS. On April 25 2007, Sony announced that a new version of Folding@home would be released the next day. The new version would improve folding performance beyond the current capacity, far beyond even the 400 teraFLOPS previously reached by PS3 users.[17]
On December 19, 2007, Sony again updated the Folding@home client to version 1.3 to allow users to run music stored on their hard drives while contributing. Another feature of the 1.3 update allows users to automatically shut down their console after current work is done or after a limited period of time (for example 3 or 4 hours).[18][19] Also, the software update added the Generalized Born implicit solvent model, so the FAH PS3 client gained more broad computing capabilities.
Multi-core processing client
As more modern CPUs are being released, the migration to multiple cores is becoming more adopted by the public, the Pande Group is adding symmetric multiprocessing (SMP) support to the Folding@home client in the hopes of capturing the additional processing power. The SMP support is being achieved by utilizing Message Passing Interface protocols. In current state it is being confined inside a single node by hard coded usage of the localhost.
On November 13, 2006, the beta SMP Folding@home clients for x86-64 Linux and x86 Mac OS X have been released. The beta win32 SMP Folding@home client is out as well, and a 32-bit Linux client is currently in development.[20]
Folding@home teams
A typical Folding@home user, running the client on a single PC, will likely not be ranked high on the list of contributors. However, if the user were to join a team, they would add the points they receive to a larger collective. Teams work by using the combined score of all their members. Thus, teams are ranked much higher than individual submitters. Rivalries between teams create friendly competition that benefits the folding community. Many teams publish their own stats, so members can have intra-team competitions for top spots.[21]
Development
The Folding@home project does not make the project source code available to the public, citing security and integrity concerns.[22][23] At the same time, the majority of the scientific codes used by the FAH (ex. Cosm, GROMACS, TINKER, AMBER, CPMD, BrookGPU) are largely Open-source software or under similar licenses.
A development version of Folding@home once ran on the open source BOINC framework; however, this version remained unreleased.[24]
Estimated energy consumption
A Playstation 3 has a maximum power rating of 380 Watts. As Folding@home is a CPU intensive application, it causes 100% utilisation. However, according to Stamford's own PS3 FAQ, http://www.stanford.edu/group/pandegroup/folding/FAQ-PS3.html, "We expect the PS3 to use about 200W while running Folding@Home." Therefore the total power consumption required to produce the processing power required by the project can be estimated based upon the average FLOPS per Watt. As of 2007, according to the Green500 list, the most efficient computer runs at 357.23 MFLOPS/watt[3]. One petaFLOPS equals 1,000,000,000 MFLOPSs. Therefore, the current Folding@home project, if it were theoretically using the most efficient CPUs currently available, would use at least 2.8 MegaWatts of power per petaFLOPS. This is equivalent to the power needed to light approximately 40000 standard house light bulbs (between 60 and 100 watts each), or just below the peak power output of one modern high-powered diesel-electric railroad locomotive. It is also important to consider that running a computer of any type can emit heat, which can be counterproductive in the summer, but actually helpful to assist in heating a room in the winter.
See also
Notes and references
- ^ Engadget, among other sites, announces that Guinness has recognized FAH as the most powerful distributed cluster, Oct 31, 2007. Retrieved Nov 5, 2007
- ^ a b c d e f "Client Statistics by OS". Folding@home distributed computing. Stanford University. 2006-11-12 (updated automatically). Retrieved 2008-01-05.
{{cite web}}: Check date values in:|date=(help) - ^ Vijay Pande (2006). "Folding@home distributed computing home page". Stanford University. Retrieved 2006-11-12.
- ^ "Validity of Folding@home" (Blog). Folding@home support forum. Stanford University. Retrieved 2006-11-12.
- ^ "Futures in Biotech 27: Folding@home at 1.3 Petaflops" (Interview, webcast).
- ^ a b "Folding@home - About" (FAQ).
- ^ a b Vijay Pande and the Folding@home team (2007). "Folding@home - Papers". Folding@home distributed computing. Stanford University. Retrieved 2007-12-13.
- ^ C. Snow, H. Nguyen, V. S. Pande, and M. Gruebele. (2002). "Absolute comparison of simulated and experimental protein-folding dynamics". Nature. 420 (6911): 102–106. PMID 12422224.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vijay Pande (2005-10-16). "Folding@home with QMD core FAQ" (FAQ). Stanford University. Retrieved 2006-12-03. The site indicates that Folding@home uses a modification of CPMD allowing it to run on the supercluster environment.
- ^ a b "Cores - FaHWiki" (FAQ). Retrieved 2007-11-06.
- ^ "FAH & QMD & AMD64 & SSE2" (FAQ).
- ^ Folding@home: Crossing the petaflop barrier
- ^ Folding@home: Post petaflop
- ^ "What is the state of Google Compute client?" (Blog). Folding@home support forum. Stanford University. Retrieved 2006-11-12.
- ^ Folding@home: Misc GPU comments
- ^ Vijay Pande (2006-10-22). "PS3 FAQ". Stanford University. Retrieved 2006-11-13.
{{cite web}}: Check date values in:|date=(help) - ^ "PS3 Folding Kicking Ass, Getting Update".
- ^ "Folding@home™ for PLAYSTATION®3 Version 1.3". Retrieved 2007-12-31.
- ^ Rimon, Noam (2007-12-18). "New Folding@home Features Coming". Retrieved 2007-12-31.
- ^ Vijay Pande (2006-11-13). "Folding@home SMP Client FAQ". Stanford University. Retrieved 2006-11-13.
- ^ Folding-community: why have teams?
- ^ "Why not OpenSource?".
- ^ "Folding@home Open Source FAQ".
- ^ "FAH on BOINC". Folding@home high performance client FAQ.
- M. R. Shirts and V. S. Pande. (2000). "Screen Savers of the World, Unite!". Science. 290: 1903–1904.
- C. Snow, H. Nguyen, V. S. Pande, and M. Gruebele. (2002). "Folding of a bba protein: simulation and theory". Nature. 420: 102–106.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - C. D. Snow, E. J. Sorin, Y. M. Rhee, and V. S. Pande. (2005). "How well can simulation predict protein folding kinetics and thermodynamics?". Annual Reviews of Biophysics. 34: 43–69.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - L. T. Chong, C. D. Snow, Y. M. Rhee, and V. S. Pande. (2004). "Dimerization of the p53 oligomerization domain: Identification of a folding nucleus by molecular dynamics simulations". Journal of Molecular Biology. 345: 869–78.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - I. Suydam, C. D. Snow, V. S. Pande and S. G. Boxer. (2006). "Electric Fields at the Active Site of an Enzyme: Direct Comparison of Experiment with Theory". Science. in press.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Folding-community: How can you tell the true nature of a Work Unit
- Folding-community: Vijay - No need to report EUEs
