Water

| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
water, oxidane
| |||
| Other names | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| Beilstein Reference | 3587155 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.028.902 | ||
| Gmelin Reference | 117 | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| Properties | |||
| H 2O | |||
| Molar mass | 18.01528(33) g/mol | ||
| Appearance | White crystal-like solid, almost colorless liquid with a hint of blue, colorless gas | ||
| Odor | None | ||
| Density | Liquid:[3] 0.9998396 g/mL at 0 °C 0.9970474 g/mL at 25 °C 0.961893 g/mL at 95 °C Solid:[4] 0.9167 g/ml at 0 °C | ||
| Melting point | 0.00 °C (32.00 °F; 273.15 K) [a] | ||
| Boiling point | 99.98 °C (211.96 °F; 373.13 K) [5][a] | ||
| N/A | |||
| Solubility | Poorly soluble in haloalkanes, aliphatic and aromatic hydrocarbons, ethers.[6] Improved solubility in carboxylates, alcohols, ketones, amines. Miscible with methanol, ethanol, propanol, isopropanol, acetone, glycerol, 1,4-dioxane, tetrahydrofuran, sulfolane, acetaldehyde, dimethylformamide, dimethoxyethane, dimethyl sulfoxide, acetonitrile. Partially miscible with Diethyl ether, Methyl Ethyl Ketone, Dichloromethane, Ethyl Acetate, Bromine. | ||
| Vapor pressure | 3.1690 kilopascals or 0.031276 atm[7] | ||
| Acidity (pKa) | 13.995[8][9][b] | ||
| Basicity (pKb) | 13.995 | ||
| Conjugate acid | Hydronium | ||
| Conjugate base | Hydroxide | ||
| Thermal conductivity | 0.6065 W/(m·K)[12] | ||
Refractive index (nD)
|
1.3330 (20 °C)[13] | ||
| Viscosity | 0.890 cP[14] | ||
| Structure | |||
| Hexagonal | |||
| C2v | |||
| Bent | |||
| 1.8546 D[15] | |||
| Thermochemistry | |||
| Std enthalpy of formation ΔfH |
−285.83 ± 0.04 kJ/mol[6][16] | ||
| Standard molar entropy S |
69.95 ± 0.03 J/(mol·K)[16] | ||
| Specific heat capacity, C | 75.385 ± 0.05 J/(mol·K)[16] | ||
| Hazards | |||
| Main hazards | Drowning Avalanche (as snow)
| ||
| NFPA 704 |
| ||
| Flash point | Non-flammable | ||
| Related compounds | |||
| Other cations | {{{value}}} | ||
| Related {{{label}}} | {{{value}}} | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||

Water (H
2O) is a simple chemical compound made of two hydrogen atoms and one oxygen atom. It is clear, has no taste or smell, and is almost colorless. All living things need water to survive.[17] Water molecules stick together because of hydrogen bonds. These bonds give water special properties. For example, water has high surface tension, and can dissolve many substances. Water exists in three forms on Earth: solid (ice), liquid (water), and gas (water vapor). The word "water" comes from the Old English word wæter.[18]
About 71% of Earth’s surface is covered by water. Most of this water (about 97%) is in the oceans. This water is salt water, which we cannot drink or use for farming. Only 3% of Earth’s water is fresh water, and even that is not easy to get. 69% of all fresh water is frozen in glaciers and ice caps. 30% is stored underground in aquifers. Less than 1% is in lakes, rivers, and swamps. If you look at all the water on Earth, only about 1% can be used by us. And most of that water is found underground.[19][20] Water is always moving in a cycle. This is called the water cycle. It includes:[21][22]
- Evaporation (water turns into vapor),
- Transpiration (plants release water vapor),
- Condensation (vapor forms clouds),
- Precipitation (rain or snow falls),
- Runoff (water returns to oceans and lakes).
Water has many unique abilities.[23] One is that it expands when it freezes. This makes ice less dense than liquid water, so ice floats.[24][25] Water also has a high specific heat capacity. It can absorb or lose a lot of heat without changing temperature much.[26] This helps keep Earth's climate stable.[27] Water’s surface tension lets it form droplets. It also lets small insects walk on water.[28] Water is an excellent solvent. It can dissolve more substances than any other liquid. This is why it is called the "universal solvent." However, it cannot dissolve oily or nonpolar substances well.[29][30][31][32] These special abilities come from water's polar nature and the hydrogen bonds between them.[33]
Water is important for all living things. Every kind of life we know needs water to survive. This includes tiny living things like bacteria, archaea, and protists, and bigger ones like fungi, plants and animals.[34] The human body is about 50-60% water.[35] Water helps move nutrients, gases, and waste inside the body. Inside cells, water is where most chemical reactions happen. It also helps cells keep their shape. Water is needed for digestion in animals, and photosynthesis in plants.[36] It also helps control body temperature. Without water, life could not exist. That is why water is one of the most important substances for all living things.[37]
Water is also very important to the world economy. About 70% of the fresh water people use goes to farming.[38] Water is used to grow crops and raise animals.[39] Fish from oceans, lakes, and rivers are an important source of food.[40] Many products, like crude oil and other goods, are moved around the world by ships through oceans, rivers, and canals.[41] Water is also used to heat and cool buildings and machines. Because it can dissolve many substances, it is very useful in factories, cooking, and cleaning. Water can also be used to make electricity, using hydroelectric plants.[42] It is also used for fun activities like swimming, boating, fishing, diving, ice skating, snowboarding, and skiing.[43]
Many civilizations in history began near rivers or other places where they could get water easily. For example, Mesopotamia began between the Tigris and Euphrates rivers.[44] Ancient Egypt depended on the Nile.[45] The Indus Valley civilization started near the Indus River.[46] Ancient Rome was built near the Tiber River.[47] Even today, many big cities like London, New York, and Shanghai grew near water.[48] Being close to rivers or oceans made it easier to trade and travel.[49][50] Islands with good harbors, like Singapore, also became rich and powerful because ships could stop there easily.[51] In dry places like North Africa and the Middle East, clean water has always been important. Having water often made the difference between a small village and a strong civilization.[52]
Water is not just found on Earth. Scientists have discovered water in many places in space. Ice has been found on Mars, and even on the Moon.[53] Some moons in our solar system, like Europa and Ganymede (around Jupiter), and Enceladus (around Saturn) may have liquid water under their icy surfaces.[54][55][56][57] Water vapor has also been found in the atmosphere of some exoplanets. These are planets that orbit stars far away from our solar system.[58][59] Water has even been found in clouds of gas and dust in space where new stars are being born.[60]
History
[change | change source]In the Universe
[change | change source]
Shortly after the Big Bang, around 13.8 billion years ago, the universe was a hot, dense soup of particles. After 380,000 years, the universe expanded and cooled down enough for the first atomic nuclei to form in a process known as Big Bang nucleosynthesis. This produced mostly hydrogen, with smaller amounts of helium and trace amounts of lithium and beryllium. Oxygen and heavier elements did not yet exist, meaning water could not yet form.[61][62]
Hundreds of millions of years later, the first stars were made from clouds of hydrogen and helium. Inside these stars, nuclear fusion began to produce heavier elements. In massive stars, fusion of lighter elements created heavier elements like carbon, nitrogen, and finally oxygen (a key ingredient for water). When these massive stars reached the end of their lives, they exploded as supernovae. This scattered those elements, including oxygen, into the interstellar medium.[63]
Now that hydrogen and oxygen existed in the universe, they began to react and form water (H₂O). This happened mainly in molecular clouds. Here, water could form in two ways. On dust grains, hydrogen and oxygen atoms stuck to the surface can react to form water ice. In hot places inside the molecular cloud like around young stars, hydrogen and oxygen gas could react to form water vapor.[64][65]
As molecular clouds collapsed to form new stars, protoplanetary disks (flat disks of gas and dust moving around the star) formed around these young stars. Water in these disks could be found as water vapor or ice. This depended on how close or far away the water was from the star. The "snow line" is a place far away from the star where it is cold enough for water to freeze and turn into ice. Inside this line, water stays as a gas, but beyond it, water becomes ice. This ice allowed particles to clump together and form planets. Water got trapped in icy planetesimals, comets, and asteroids. In the inner parts of star systems, it was usually too warm for ice, so most water there was in the form of vapor. Some water may have arrived later when comets or water-rich asteroids from the colder outer regions crashed into these inner planets, bringing ice that had formed far from the star.[66][67]
On some planets and moons, the conditions were just right for water to exist as a liquid, solid (ice), or gas (vapor). Earth is the best and only example we know of. Because it is the right distance from the Sun and has a protective atmosphere, it has had oceans of liquid water for billions of years. Other places, like the moons Europa, Enceladus, and Ganymede, have oceans hidden under thick layers of ice. Mars has signs that it once had rivers and lakes long ago. Comets and asteroids still hold water from the early days of the solar system.[68]
Today, water continues to be everywhere but mostly frozen or in vapor form. It found in molecular clouds, where stars and planets are still being made. It can also be found in interstellar space as ice on dust grains. It has also been found in the atmospheres of exoplanets. At the center of galaxies, massive amounts of water, billions of times the amount on Earth has been found around black holes. But, liquid water remains extremely rare, because it requires a very narrow set of conditions. Earth is unique because it contains liquid water on its surface.[69]
On Earth
[change | change source]
One of the biggest mysteries in astronomy is where Earth's water came from. Scientists are still trying to answer this question. Earth is special because it is the only rocky planet in our Solar System with oceans of liquid water on its surface. The other rocky planets, Mercury, Venus, and, Mars do not have large amounts of liquid water on their surface.[70] Liquid water is important because every living thing we know needs it to survive. Earth has liquid water because it is in just the right spot in space. This spot is called the habitable zone. It is not too close to the Sun, where water would evaporate. It is also not too far away, where water would freeze. This perfect distance helps keep water in its liquid form on Earth.[71]
There are many ideas about how Earth got its water. Most of these ideas fall into two main groups. One idea is that Earth already had the right ingredients (hydrogen and oxygen) when it formed, and those combined to make water. The other idea is that water came from space. It was carried by asteroids or comets that crashed into Earth after it formed. Some scientists think both of these ideas could be true. Earth might have gotten its water from more than one source. That makes the mystery a bit more complex, but researchers are trying their best to understand what happened in the early Solar System and how water ended up on our planet.[72][73][74]
Earth formed about 4.54 billion years ago, after the Sun was born from a huge cloud of gas and dust. The leftover material from that cloud became the rest of the Solar System, including Earth. One idea is that Earth was born with everything it needed to create oceans, lakes, and rivers. But there is a problem. The early Solar System was extremely hot, especially near the center where Earth formed. Any water on the surface would have turned into gas and escaped into space.[75] Another idea is that Earth’s water may have come from deep inside the planet. Water might have been trapped inside rocks deep underground when Earth was forming. This water was safe from the heat because it was hidden inside minerals in Earth's mantle. Over time, volcanoes released that water as steam. When the steam cooled, it turned into rain that helped form oceans and rivers.[76]
Another idea is that Earth may have created its own water. Powerful space telescopes have seen young planets far away, that are surrounded by thick clouds of hydrogen gas. Scientists think that when Earth was very young, it might have had a lot more hydrogen in its atmosphere than it does today. Right now, Earth’s atmosphere is mostly nitrogen, and has almost no hydrogen. One idea suggests how hydrogen gas from the atmosphere could react with molten rock (magma) on the early Earth. This reaction could have produced large amounts of water. As the surface cooled and hardened, this water would have stayed on the planet, forming oceans and lakes.[77]
A long time ago, the inner Solar System was a dangerous place. Between 4.0 and 3.8 billion years ago, the outer planets (Jupiter and Saturn) moved around and changed their orbits. Their powerful gravity sent icy space rocks flying toward the inner planets, including Earth. This time period is called the Late Heavy Bombardment. Many of these space rocks crashed into Earth, and scientists think they could have brought water with them. At first, people thought the water mainly came from comets. But space missions like Giotto (which visited Halley's Comet in 1986) and Rosetta (which visited another comet from 2014 to 2016) found that the water on comets is different from the water in Earth’s oceans. That means comets probably weren’t the main source. Instead, scientists now think asteroids and meteorites brought most of the water. For example, a spacecraft called Hayabusa2 brought back pieces of an asteroid named Ryugu. Scientists found that the water trapped in its rocks looks like Earth’s ocean water. Ryugu is made of the same stuff as a type of meteorite called CI chondrites, which could have delivered up to 30% of the water in Earth’s oceans.[78][79][80][81]

Scientists use ancient rocks to figure out when water first appeared on Earth. One kind of rock they use is called pillow basalt, which forms when a volcano erupts underwater. One of these rock was found in the Isua Greenstone Belt in Greenland. It is about 3.8 billion years old. This tells us that there was liquid water on Earth at that time.[82] More of these very old rocks can be found in Canada, in a place called the Nuvvuagittuq Greenstone Belt. Some studies of these rocks also say there was water about 3.8 billion years old. Other studies suggest there was water 4.28 billion years.[83] If there was water, like oceans, even earlier than that, we don’t know yet. That might be because the Earth’s surface is always changing over time. Old rocks can be destroyed or buried through processes like plate tectonics and recycling of the crust, which can erase early signs of water. In 2020, scientists suggested that there might have been enough water to fill the oceans immediately after Earth formed.[84][85][86]
To understand what Earth was like after it formed, scientists study rocks called zircons. Unlike most rocks, zircons are very tough. They can survive for billions of years, making them useful for studying early Earth. Zircons show that there was liquid water and an atmosphere 4.404 billion years ago, not long after Earth formed.[87][88][89][90] This creates a problem, because the cool early Earth hypothesis says that Earth was cold enough to freeze water between 4.4 and 4.0 billion years ago.[91] Other zircon studies from ancient Australian rocks suggest that plate tectonics may have started around 4 billion years ago.[92] If that's true, Earth may have been similar to today, instead of being hot and covered in carbon dioxide. Plate tectonics helps trap carbon dioxide, which cools the planet and allows solid rock and liquid water to form.[93]
In human civilization
[change | change source]
In early human history, people survived by hunting animals and gathering plants. They often moved around and stayed close to sources of water or carried water around. But about 12,000 years ago, during the Neolithic revolution, humans started farming and raising animals. To do this, they needed a steady supply of water. Areas with rich soil and regular water made farming possible. This let people settle in one place and live in larger groups. Water also provided food, like fish and other water animals, and helped people travel using simple boats like canoes.[94][95][96][97]
The first human civilizations began in river valleys, where there was plenty of fresh water and good soil for farming. For example, Mesopotamia, known as the "Cradle of Civilization," grew between the Tigris and Euphrates rivers. People there built irrigation canals to bring water to their crops.[98][99] In Ancient Egypt, the Nile river was very important. It helped people grow food, raise animals, and move goods and people around.[100][101] The Indus Valley civilization grew near the Indus River. They built advanced water systems, like wells, drains, and public baths.[102][103] In Ancient China, the Yellow River helped northern China grow, but it also flooded a lot and caused many deaths. That is why it was called both "China’s pride" and "China’s sorrow."[104][105][106] In Ancient Rome, people built aqueducts. They were long channels that carried water from far away places into cities. This water was used for farming, public baths, toilets, and even homes.[107][108][109]
In the earliest civilizations, rivers and seas were very important for transport and trade. They made it easier to move heavy goods over long distances. In Mesopotamia, the Tigris and Euphrates rivers connected cities like Ur and Babylon.[110][111] In Egypt, the Nile River helped people trade from Nubia in the south all the way to the Mediterranean Sea.[112][113][114] The Phoenicians were great sailors who built strong ships and set up trade colonies around the Mediterranean. Busy ports like Alexandria, Athens, Tyre, and Carthage became rich and full of culture because of sea trade.[115][116][117][118] In Medieval Europe, rivers like the Danube, Rhine, and Seine helped create strong trade networks.[119][120] Cities like Venice and Genoa became powerful by trading across the sea.[121][122] In the 1400s, countries like Portugal, Spain, England, and the Netherlands began exploring the world by sea. Christopher Columbus’s voyages opened up the Atlantic Ocean.[123][124] This connected Europe, Africa, and the Americas in what became known as the Columbian Exchange. Later, the British and the Dutch East India Company built huge trade empires using powerful ships and ocean routes.[125][126]
As cities got bigger with more people, their water systems could not keep up with the growing population. This led to more disease outbreaks. This was before people understood how germs worked. Diseases like typhoid, cholera, and dysentery spread quickly through dirty water and killed millions of people over the centuries.[127] In Medieval Europe, cities did not have good plumbing. Waste flowed through open sewers in the streets and often mixed with drinking water. This caused many deadly epidemics. In the 1800s, London had several major cholera outbreaks. At first, people thought the disease came from bad air, called “miasma.” But in 1854, a doctor named John Snow proved that cholera was actually spread through dirty water. He traced the outbreak to a single water pump on Broad Street. People learned that having water was not enough. It had to be clean and kept away from waste. In the late 1800s and early 1900s, cities began improving public health by building better water systems. Engineers created piped water networks, storage tanks, and pumping stations to bring clean water to homes. They also built underground sewer systems to safely carry away human waste. Cities like London, Paris, and New York only made these changes after big epidemics showed how serious the problem was. New water treatment methods like sand filtering and adding chlorine helped kill germs and made water safer to drink.[128][129][130]
A long time ago, ancient Greek thinkers like Thales of Miletus believed that water was the basic building block of everything in the world.[131] Around 250 BCE, Archimedes used water to figure out the volume of an object by seeing how much water it pushes away when put in water.[132] During the Renaissance, Leonardo da Vinci studied how water flows and how it helps wear away rocks over time. He helped build canals and other things to help control water.[133] Galileo Galilei looked at why some things float on water and others do not.[134] He also studied tides, the regular rising and falling of the sea.[135] By the late 1700s, Antoine Lavoisier figured out that water was not an element, but a compound made from hydrogen and oxygen.[136]
Today, about 70% of all the freshwater we use around the world goes to agriculture, making farming the biggest user of water. Plants need water to grow, animals need it to live, and irrigation helps farmers grow crops even in dry areas.[137] In modern times, how much water a country has can affect whether it can grow enough food for its people or if it has to buy food from other countries. Places with lots of water, like the United States, Brazil, China, and India, are major food producers. These countries grow extra crops like wheat, corn, soybeans, and rice, and sell them to other countries. Having lots of rain, rivers, underground water, and good systems like dams and canals gives these countries a big advantage.[138] But countries with very little water, like Saudi Arabia, Qatar, the UAE, and Kuwait have trouble growing enough food. They do not have enough freshwater or good land for farming, so they import most of their food from other places.[139] Fish are also an important source of food for the world. Over 1 billion people depend on fish as their main source of protein, especially in places near rivers and oceans.[140] Fish farming, called aquaculture, has allowed us to raise fish instead of looking for them.[141]
Even today, oceans and rivers are still the main way we move goods around the world. In fact, about 80% of all global trade by volume travels by sea.[142] Oceans, rivers, and canals are great for moving things like fuel, raw materials, and products because it is cheaper than using trucks or planes.[143] Some water routes are especially important. The Suez Canal, Panama Canal, Strait of Hormuz, and Strait of Malacca are key paths for global trade.[144] Large ports like those in Shanghai, Rotterdam, Singapore, and Los Angeles help grow their countries' economies.[145][146] Big rivers like the Mississippi in the U.S., the Yangtze in China, and the Danube in Europe are also important. They help move large amounts of goods inside countries cheaply.[147][148][149]
Modern water systems help bring clean water to billions of people around the world. Dams and reservoirs store water for drinking, farming, flood control, and even to make electricity. Famous examples include the Hoover Dam in the U.S. and the Three Gorges Dam in China.[150] Pipelines and aqueducts move water from places that have a lot of it to places that do not have much and big cities. For example, Los Angeles gets much of its water from places far away.[151] In very dry regions like the Middle East, Israel, and parts of Australia, desalination plants turn salty seawater into fresh drinking water. But this process uses a lot of energy.[152] Wastewater treatment plants clean dirty water from homes and businesses so it does not pollute rivers and lakes. Sometimes, this cleaned water is reused for farming or industry.[153]
The world still faces big problems with water. More than 2 billion people live in places where there is not enough water.[154] In areas like India, the southwestern U.S., and the Middle East, people are using up groundwater faster than it can be replaced. Because of this, there might not be enough water in the future.[155] Climate change is making things worse. It causes longer droughts, changes where and when rain falls, and reduces the amount of snow in mountains. Snow is important because it slowly melts releasing water into rivers over time.[156] Pollution is also a big problem. Factories, farms, and untreated sewage can make rivers and lakes dirty.[157] Rising sea levels are pushing saltwater into underground water supplies near the coasts, making them unsafe to drink.[158] More powerful storms are causing floods that city drainage systems can not handle.[159] Because of this, we must protect and manage water carefully for the future.
Properties of water
[change | change source]

Water is a chemical substance. It is made of two hydrogen atoms and one oxygen atom. Its chemical formula is H₂O. These atoms are held together by something called a covalent bond. At room temperature and normal pressure, water is a liquid. It has almost no color, taste or smell. Water is often called the "universal solvent" because it can dissolve more substances than any other liquid. But it cannot dissolve nonpolar substances like oil very well. Water is also the only common material on Earth that can naturally exist as a solid, liquid, and gas.[33]
A water molecule looks like a bent “V” shape. The angle between the two hydrogen atoms is about 104.5°. This shape happens because of how electrons are arranged around the oxygen atom. Oxygen has six outer (valence) electrons. It uses two of them to bond with two hydrogen atoms (one for each). The other four electrons stay in two pairs, called lone pairs, that do not bond with anything. According to VSEPR theory, all these pairs of electrons want to stay as far apart as possible. This creates a shape like a tetrahedron with four regions: two bonding pairs and two lone pairs. But lone pairs take up more space and push harder than bonding pairs. They push the hydrogen atoms closer together, so the angle becomes smaller than the usual 109.5° of a perfect tetrahedron. That is why the water molecule is bent instead of straight, and why the bond angle is 104.5°.[160][161][162]
Hydrogen bonds
[change | change source]
A water molecule (H₂O) has one oxygen atom and two hydrogen atoms. Oxygen is much more electronegative than hydrogen. This means it attracts electrons closer to itself much more than hydrogen. So, when oxygen and hydrogen share electrons in a water molecule, the electrons spend more time near the oxygen atom. This gives the oxygen atom a partial negative charge (δ⁻). And the hydrogen atoms gains a partial positive charge (δ⁺). So, the water molecule now has a positive side and a negative side. Because of this, the positive hydrogen of one water molecule is attracted to the negative oxygen of another. This attraction is called a hydrogen bond. It is not as strong as a normal chemical bond (like a covalent bond). But, it is strong enough to hold water molecules loosely together. Hydrogen bonding explains many of water’s unusual properties. For example, water's high boiling and melting points, high surface tension, the ability of ice to float on water, and the ability to dissolve many substances.[160][163]
Water (H₂O) is a liquid at room temperature. This might seem normal until you compare it to similar substances. Water belongs to a group of compounds called hydrogen chalcogenides. These are made when hydrogen bonds with elements from the same family as oxygen: sulfur, selenium, and tellurium. This includes hydrogen sulfide (H₂S), hydrogen selenide (H₂Se), and hydrogen telluride (H₂Te). But, while water is a liquid, the others are all gases at room temperature. The reason is hydrogen bonding. Oxygen is very electronegative (it strongly pulls on electrons) and is small in size. Because of this, water molecules can form strong hydrogen bonds. Each water molecule can form up to four hydrogen bonds with its neighbors. This creates a kind of "stickiness" that holds the molecules together and makes it harder for them to break apart and turn into a gas. That is why water has a high boiling point of 100°C (212°F) for such a small molecule. The other chalcogens (sulfur, selenium, and tellurium) are larger but less electronegative than oxygen. In hydrogen sulfide (H₂S), hydrogen selenide (H₂Se), and hydrogen telluride (H₂Te), the bonds between hydrogen and the other elements are weaker. These molecules do not form strong hydrogen bonds. Instead, they only have weak van der Waals forces, which are easy to break. So these substances boil at much lower temperatures. Hydrogen sulfide (H₂S) boils at –60°C. Hydrogen selenide (H₂Se) boils at –41.5°C. Hydrogen telluride (H₂Te) boils at –2.2°C. That is why they are gases at room temperature, while water is a liquid.[164][165][166][167]
Cohesion and adhesion
[change | change source]

Water molecules stick to each other. This is called cohesion. This happens because each water molecule can form hydrogen bonds with its neighbors. Inside a drop of water, each molecule is pulled in all directions by the other water molecules around it. But at the surface, there are no water molecules above. The hydrogen bonds at the surface of water pulls molecules tightly together, forming a sort of "skin" on the surface. This is called surface tension. This is why water forms droplets. It is also why small insects, like water striders, can walk on water. It is also why small objects like a needle can float if placed gently on water.[168][37][169]
Water molecules can stick to other surfaces. This is called adhesion. This especially happens to surfaces that are polar or charged, like glass, plant cell walls, or soil particles. Water's adhesion can be seen when water climbs up the side of a glass container, forming a curved surface called the meniscus. It can also be seen as water spreads out on a leaf or sticks to surfaces like spider webs. Whether water forms flat puddles or round beads depends on the balance between cohesion and adhesion. If water sticks more to the surface, it spreads out. If water sticks more to itself, it forms beads.[170][171][172]
Water can climb up a thin tube or narrow space. This is called capillary action. It happens because adhesion pulls water molecules to the sides of a thin tube or pore, while cohesion pulls other water molecules along with it.[173] In plants, capillary action helps water move from the roots to the leaves. As water evaporates from the leaves, it pulls more water up behind it. Adhesion helps the water stick to the sides of the plant's tubes. Cohesion keeps the water molecules connected like a chain, so they move upward together. This allows them to move up against gravity.[174][175]
Heat and water
[change | change source]
Water is very good at staying at the same temperature. It does not heat up or cool down quickly. This is because water has a high specific heat capacity. That means it takes a lot of heat to make water warmer, and it cools down slowly too. Specific heat is the amount of heat needed to change the temperature of 1 gram of a substance by 1°C. For water, this is 1 calorie. That is much higher than most other common substances. For example, alcohol only needs 0.6 calories to do the same, so it heats up faster than water.[176]
If you touch a metal pot with warm water inside, the metal might feel hotter than the water. That is because metal heats up faster than water. Water takes longer to warm up because water molecules are held together by hydrogen bonds. When heat is added, much of the energy goes into breaking the hydrogen bonds between water molecules. Once those bonds are broken, the molecules can move faster, and the temperature rises. So instead of heating up quickly, water stores heat by breaking the hydrogen bonds first. When water cools down, the hydrogen bonds form again and release heat. This helps keep temperatures steady. Oceans and lakes can absorb heat from the sun during the day or in summer and release it slowly at night or in winter. That is why places near water usually have milder temperatures. Water’s high specific heat also keeps the ocean from getting too hot or too cold. This is very important for ocean life. And because our bodies are mostly made of water, this also helps us keep a steady body temperature.[27][37][177]
Water molecules usually stay close together. But if some molecules move fast enough, they can break free and escape into the air as gas. This process is called evaporation. Even at low temperatures, the fastest molecules can still escape. This is why a glass of water will slowly disappear over time. Heating water makes its molecules move faster, so it evaporates faster too. The heat of vaporization is the amount of heat needed to turn 1 gram of liquid water into gas. For water, it takes about 580 calories to evaporate just 1 gram at 25°C. This is nearly double the amount needed for ammonia and alcohol. Water’s high heat of vaporization is because of hydrogen bonds. Water molecules are held together through hydrogen bonds. To vaporize water, these bonds must be broken, which takes a lot of energy. The energy does not raise the temperature. It goes into breaking the hydrogen bonds. That is why water takes a long time to boil and releases a lot of heat when it condenses.[178][37]
This high heat of vaporization has important effects on Earth. Tropical oceans take in a lot of heat from the Sun. Some of this heat is used to evaporate water. When that water vapor travels to cooler places and turns back into rain, it releases heat. This helps balance Earth’s climate and spread heat around the planet.[179] Evaporation also cools things down. When water evaporates, the fastest (hottest) molecules leave first. This lowers the temperature of the water that is left behind. This is called evaporative cooling. It helps keep lakes, plants, and animals from getting too hot. When we sweat, the sweat takes heat from our skin as it evaporates. This is why sweating cools down. On hot, humid days, the air already has a lot of water vapor. That makes it harder for sweat to evaporate, so we feel even hotter.[180] Some animals that cannot sweat, like elephants, cool themselves by spraying water on their skin.[37][181]
It also takes a lot of energy to melt ice. This is called the heat of fusion. The heat of fusion is the amount of energy needed to change 1 gram of a substance from solid to liquid at its melting point. For water, this is 334 joules per gram (J/g). That is higher than most other substances, especially for a molecule as small as water. When water freezes into ice, its molecules form a crystalline structure held together by hydrogen bonds. To melt ice, you need enough energy to break many of these bonds. The heat added goes into breaking these bonds, but does not increase the temperature. The water that is produced also remains at 0°C until all of the ice is melted. The same amount of energy needed to melt ice could warm that same ice from –160°C all the way up to 0°C. Ice and snow can take in heat without melting right away. This helps keep temperatures from changing too quickly. In cold places like the North and South Poles or high mountains, this helps keep the temperature more steady.[182] Before we had refrigerators, people used ice to keep food cool because it stayed frozen for a long time.[183][184][185]
Density of ice
[change | change source]
Water is one of the only substances that becomes less dense when it freezes. This means that ice floats on water. Most substances get smaller and denser when they freeze. But water is different. It expands (or gets bigger) when it freezes. This strange behavior happens because of hydrogen bonds. At temperatures above 4°C, water acts like most liquids. It expands when heated and shrinks when cooled. But between 4°C and 0°C, something strange happens. As the water gets colder, the molecules slow down. They do not have enough energy to break the hydrogen bonds between them. At 0°C, the molecules line up into a solid crystal shape. Each molecule connects to four others with hydrogen bonds. These bonds hold the molecules further apart. So the ice takes up more space than the liquid water, even though it has the same number of molecules. That is why ice is about 9% less dense than water at 4°C. When ice melts, the bonds break and the molecules move closer together. Water is densest at 4°C. It becomes less dense if it gets warmer or cooler from that temperature.[186][24]
The fact that ice floats is very important for life on Earth. If ice sank, lakes and oceans could freeze all the way to the bottom. This would make it hard or even impossible for fish and other living things to survive. Instead, ice floats on top, and acts like a blanket. This helps keep the water below from freezing. Fish and other organisms can stay alive under the ice. Also, floating ice provides homes for animals like polar bears and seals.[186][37]
Water as a universal solvent
[change | change source]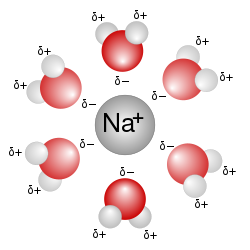
When you drop a sugar cube into a glass of water, the sugar slowly dissolves and spreads out. This creates a mixture. This type of mixture is called a solution. In a solution, the solvent is the substance that does the dissolving (in this case, water), and the solute is the substance that gets dissolved (the sugar). If water is the solvent, the solution is called an aqueous solution.[29][31][37]
Water is a very good solvent. Because of this, it is often called the "universal solvent". This is because it can dissolve more substances than any other substance. This happens because water molecules have slightly positive and negative parts. These parts attract other charged or polar substances. For example, when you add salt (sodium chloride, or NaCl) to water, it breaks into sodium (Na⁺) and chloride (Cl⁻) ions. The negative side of water pulls on the sodium, and the positive side pulls on the chloride. Water molecules surround each ion. They then pull them away from the salt crystal. After that, they spread them out in the water. This group of water molecules around an ion is called a hydration shell. Other ionic compounds, like potassium chloride, also dissolve in water this way. Seawater is full of dissolved ions like these. But a substance does not have to be made of ions to dissolve in water. Polar molecules like sugar can also dissolve. This is because they can form hydrogen bonds with water. Even large molecules, like proteins, can dissolve in water if they have polar or charged areas on their surfaces.[29][30][32][37]
Water is the main solvent for living things. Many important substances are dissolved in the water in blood, plant sap, and cells. Any substance that mixes well with water is called hydrophilic (water-loving). Some hydrophilic substances do not dissolve in water. For example, some molecules in cells are too big to dissolve. Another example is cotton. Cotton is made up of giant molecules of cellulose. Cellulose does not dissolve in water. It has lots of positive and negative parts that can form hydrogen bonds with water. Water sticks to the cellulose. That is why cotton towels drys things well, but does not dissolve in the washing machine. Plants also use cellulose in the tubes that carry water from roots to leaves. Water sticks to the hydrophilic walls of these tubes, helping it move upward against gravity. On the other hand, substances that do not mix with water are called hydrophobic (water-fearing). These include oils and fats. They don’t dissolve because they do not have charges that attract water. That is why oil and water do not mix. Hydrophobic molecules can be found in cell membranes. Without them, cells would dissolve in water. Life would be impossible.[37][31][30]
States of water
[change | change source]
Water can exist in three main forms: solid, liquid, and gas. These are called states or phases. Which one water is in depends on the temperature and pressure.
When people say "water" in everyday life, they usually mean liquid water. This is the form that comes from taps, fills oceans, rivers, and lakes, and is used for drinking, cooking, and cleaning. Liquid water is the most common form found on Earth's surface. When water gets cold enough, it freezes and becomes a solid called ice. Ice can be in the form of hard cubes (like in your freezer) or soft, loose crystals, like snow. There are also other strange kinds of ice. These are often found in extreme environments like outer space deep inside Uranus. When water gets hot enough, it turns into a gas called water vapor. This is what we see as steam rising from boiling water.
Water can also exist in a very strange state called a supercritical fluid. This only happens at extremely high temperatures above (374°C or 705°F) and very high pressures (above 22.064 megapascals). Here, water acts like a gas and a liquid at the same time. It can flow like a liquid and spread out like a gas. Supercritical water is useful because it can dissolve many things that normal water cannot. It can dissolve nonpolar organic compounds like oil. This strange state of water does not happen naturally on Earth’s surface. But it can happen deep in the ocean. One example is near hydrothermal vents. This happens at around 2200 meters deep. The ocean is much deeper than that on average at about 3800 meters.[187]
Changing states
[change | change source]

A phase diagram is a special graph. It shows how a substance like water changes between solid, liquid, and gas depending on the temperature and pressure. On this graph, the bottom (horizontal) line shows temperature. The side (vertical) line shows pressure. The graph is divided into three parts. One part shows where water is a solid (ice). Another part shows where it is a liquid (water). And another part shows where it is a gas (steam).[188] There is a special point on the graph called the triple point. At this temperature and pressure, water can exist as a solid, liquid, and gas at the same time. For water, this happens at 0.01 °C and a pressure of 611.657 pascals.[189]
At normal air pressure (1 atmosphere), water freezes into ice at 0 °C (32 °F) and boils into steam at 100 °C (212 °F). The freezing point is the temperature at which water turns to ice. The boiling point is the temperature at which water turns into gas. Water does not need to be boiling to become gas. Even at low temperatures, some water molecules can move fast enough to evaporate. This is why a glass of water when left alone will slowly dry out. But when water is heated, the molecules move faster and evaporate quicker. When water reaches 100 °C, bubbles of water vapor form inside the liquid. These bubbles rise to the top and release steam into the air.[29]
Water vapor (gas) can turn directly into ice without becoming liquid first. This is called deposition. You can see this when frost forms on cold windows. It also happens when snowflakes form in clouds. In clouds, tiny pieces of dust or pollen help water vapor turn straight into ice.[190] The opposite of deposition is called sublimation. This is when ice turns straight into water vapor without becoming a liquid.[191] One use of sublimation is in freeze-drying food. a method of preserving food. First, the food is frozen. Then it is put into a vacuum (a space with no air). The ice inside the food turns into vapor. This leaves the food dry, without using heat.[192]
Water usually freezes at 0 °C (32 °F) at normal air pressure. But in special conditions, pure water can stay liquid even it is colder than that. If it is not shaken or disturbed, it can cool all the way down to about –42 °C (–44 °F) without freezing. This is called supercooling.[193]
The melting and boiling points of water change with pressure. For most things, if you add more pressure, they melt at a higher temperature. But water is different because ice is less dense than liquid water. That is why ice floats. When pressure is added to ice, the melting point actually goes down. That means ice can melt even when it is colder than 0 °C if there is enough pressure.[194] This can happen deep under a glacier. The heavy ice on top pushes down with a lot of pressure. This pressure can melt the ice under it, even though it is very cold. That is how lakes can form under glaciers.[195][196]
Steam (which is water in gas form) takes up much more space than liquid water. That means it is less dense. When the pressure is high, it becomes harder for water to boil, so it needs to be hotter to turn into steam. In places with a lot of pressure, water can stay as a liquid even when it gets hotter than 100 °C (212 °F), which is the normal boiling point.[197] For example, in geysers like Old Faithful, water can get over 205 °C (401 °F) without boiling.[198] And near underwater volcanoes called hydrothermal vents, water can reach 400 °C (752 °F) and still stay liquid.[199]
At sea level, water boils at 100 °C (212 °F). But when you go up higher like up the mountains, the air pressure gets lower. When pressure is lower, water boils at a lower temperature. For every 274 meters (or about 900 feet) you go up, the boiling point goes down by about 1 °C. For example, at 274 meters (about 900 feet), the boiling point becomes 99°C (210.2°F) instead of 100°C (212°F). That is why food takes longer to cook at higher altitudes. The water boils before it gets really hot.[200] A pressure cooker works the opposite way. It traps steam inside, which raises the pressure. This lets water boil at a higher temperature, so food cooks faster.[201] In places with no air at all, like in a vacuum, water can even boil at room temperature. This is because there is no pressure holding the water molecules together.[202]
Taste and odor
[change | change source]People often say that water has no taste or smell.[33] But in real life, most of the water we drink has some taste or smell. This is because there are usually tiny amounts of other substances dissolved in the water. Pure water does not have a taste, but our tongues can tell if something is mixed in. For example:
- Salts in water can give it a “mineral” taste, like water from springs.[203][204]
- Water that is very acidic tastes sour.[205]
- Water that is very basic (alkaline) tastes bitter.[206]
- Tap water often has chlorine added to kill germs. This can give it a chemical or medicine-like taste.[207]
- Metals like iron or copper can cause a metallic taste. This can be found in water from old pipes.[208]
Even tiny amounts of these substances can change the taste. How strongly people taste them can depend on how cold or warm the water is and even a person's genes.[209][210]
- Water can smell earthy or musty. This often come from natural chemicals like geosmin or 2-methylisoborneol (MIB) made by algae or bacteria in lakes and rivers.[211]
- A rotten egg smell means the water might have hydrogen sulfide gas in it.[212]
- Tap water might smell a little like chlorine, from the treatment process.
Some of these smells are so strong that the human nose can detect them even in very small amounts.[213] Some animals, like frogs, can even smell water itself.[214]
Mechanical properties
[change | change source]Water is often called incompressible. This means that even if you push on it really hard, it doesn’t shrink much. For example, at the bottom of the ocean, about 4 kilometers (2.5 miles) deep, the pressure is 400 times greater than at sea level. But water only gets about 1.8% smaller. This happens because water has a high bulk modulus (about 2.2 gigapascals). This means it resists being squeezed.[215]
Viscosity is how easily a liquid flows. Water has low viscosity, so it flows quickly and easily, like in rivers or through pipes. Honey or syrup have high viscosity, so they flow slowly and are thick. Water flows smoothly through rivers, pipes, and the human body (like in blood vessels and cells). Viscosity changes with temperature. When a liquid gets warmer, it becomes thinner and flows more easily. When it gets colder, it becomes thicker and flows more slowly. So, warm water flows faster, and cold water flows slower. Water is called a Newtonian fluid. This means its viscosity stays the same even if you stir it fast or slow.[216]
Sound travels through water at about 1,400 to 1,540 meters per second, depending on how warm, salty, or deep the water is. That is over 4 times faster than in air. Whales use sound to talk and find food underwater. Humans use tools like sonar to find things under the sea.[217][218]
Electrical properties
[change | change source]
Water is a polar molecule. This means it has slightly charged ends. In water molecules, the hydrogen atoms have a small positive charge, and the oxygen atom has a small negative charge. This happens because oxygen pulls electrons closer to itself. That is because oxygen is more electronegative. It likes to attract electrons more than hydrogen does. This polarity helps water surround and pull apart other substances, especially those made of charged particles, like table salt (NaCl). When salt is added to water, the negative part of water (the oxygen) surrounds the positive sodium ions (Na⁺). The positive part (the hydrogen) surrounds the negative chloride ions (Cl⁻). This helps break the salt apart and keep the ions floating around in the water.[30][31]
Water also has a high dielectric constant. It is about 80 at room temperature. That is much higher than most other liquids. This means water can reduce the electrical attraction between opposite charges. As a result, ions (like those from salt) can separate more easily in water. This is why it is so good at dissolving electrolytes. This makes it easier for ions to stay separate and move around freely. This is important for conducting electricity in biological and chemical systems. Thanks to its polarity and dielectric nature, water is one of the best solvents in the world. That is why it is called the "universal solvent".[219]
Water can go through a special process called autoionization (also called self-ionization). This means that two water molecules can react with each other to make two new particles: a hydronium ion (H₃O⁺) and a hydroxide ion (OH⁻). One water molecule, takes a hydrogen from the other becoming a hydronium ion while the other is left with one hydrogen becoming a hydroxide ion.[219]
The reaction looks like this:
- H2O + H2O
 H3O+ + OH−
H3O+ + OH−
This reaction does not happen often. At room temperature (25 °C), only a tiny number of water molecules do this. In pure water, the amounts of H₃O⁺ and OH⁻ are each 1 × 10⁻⁷ mol/L (moles per liter). That tiny amount makes water neutral and gives it a pH of 7. The pH scale, which tells us if something is acidic or basic, is based on this. Because there are so few ions, pure water does not conduct electricity well. That means pure water is more like an insulator than a conductor.[220]
But water can dissolve ionic substances like salt very well. When you add even a little table salt (NaCl) to water, it releases lots of ions. The conductivity goes up a million times. That is why tap water and seawater conduct electricity so well. Seawater, for example, has ions like Na⁺, Cl⁻, and Mg²⁺, making it highly conductive. This matters in real life. Distilled water, which has no ions, can’t carry electricity. But regular water, with minerals or salts in it, can carry electric current. This is important in batteries, electrolysis, and even in cells in your body.[221]
Optical properties
[change | change source]
In small amounts, water looks clear, but pure water actually has a slight blue tint. You can see the blue color more easily when you look at a large amount of water, like in a swimming pool, lake or ocean. The color comes from the way water interacts with light. Pure water absorbs some colors of light more than others. Those colors include the red, orange, and yellow parts of sunlight. This leaves more blue light to be reflected into our eyes. This is why water looks blue. You cannot see this in a glass of water. You can only see this when you are looking at deeper water, like in a swimming pool or lake. The deeper the water, the more light gets absorbed, and the more the blue color is seen. If water has something dissolved in it or tiny particles floating in it, the color can change. For example, algae and organic matter can make water look green or brown. Minerals and sediments in the water can change its color. Tannins from decaying plants can give water a tea-like, brownish color.[223]
Visible light can mostly go through water. Blue and green light go the deepest in water. On the other hand, red, orange, and yellow light gets absorbed by the water, so they do not go very deep. In clear ocean water, sunlight can reach down to about 200 meters. This upper layer is called the photic zone. It is where there is enough light for plants and tiny algae like phytoplankton to do photosynthesis. Deeper than that, there is not enough light for plants to do photosynthesis.[224]
Water bends light because it has a higher refractive index than air. The refractive index of water at room temperature (20°C or 68°F) is about 1.333. The refractive index of air is about 1.0. This means that when light enters water from the air, it slows down and bends. This is called refraction. It is this bending of light that causes a straw to look bent or broken in a glass of water. It is also why things under water look closer or larger than they actually are. Ice has a slightly lower refractive index (about 1.31). Because of this, light bends a bit less when passing through ice than through liquid water. The refractive index of water is similar to some liquids like ethanol and alkanes. But, it is lower than substances like glycerol, benzene, carbon disulfide, or glass (which range from 1.4 to 1.6). Also, the refractive index of water can change a little depending on the how how or cold it is, the pressure, or how much salt is in the water.[225]
Chemical reactions of water
[change | change source]Water can react with some metals. It reacts with metals more reactive than hydrogen. When this happens, the metal reacts with water to make hydrogen gas and a metal hydroxide. Some metals, like the alkali metals (such as lithium, sodium, and potassium), react very strongly with water. Alkaline earth metals like calcium and magnesium react less violently. For example, sodium reacts violently and makes sodium hydroxide and hydrogen gas:[226]
- 2Na + 2H2O → 2NaOH + H2
This reaction gives off a lot of heat (exothermic). It can even cause the hydrogen gas to catch fire.
Water is amphoteric. This means it can act like an acid or a base. This depends on what it is reacting with. When water is with a strong base, it gives away a hydrogen ion (H⁺) and acts like an acid. When water is with a strong acid, it takes in a hydrogen ion and acts like a base.[227]
Water can also react with itself in a process called self-ionization:
- H2O + H20 ⇌ H3O+ + OH−
This creates a hydronium ion (H₃O⁺) and a hydroxide ion (OH⁻). This reaction does not happen much in water. It helps set the pH scale, which tells us how acidic or basic a liquid is.[220]
Water can also break one molecule into two smaller ones in a process called hydrolysis. The word comes from Greek: "hydro" meaning water, and "lysis" meaning to break or unbind. In hydrolysis, one molecule breaks into two molecules. A water molecule then breaks apart into a hydrogen ion and a hydroxide ion. The hydrogen ion attaches itself to one of the two new molecules.[228] The hydroxide ion attaches itself to the other molecule. In industry, hydrolysis is used to break down compounds like esters or salts. In biology, proteins are broken into amino acids through hydrolysis. Fats (lipids) are broken into glycerol and fatty acids. Carbohydrates, such as sucrose, are broken into simpler sugars like glucose and fructose:
- C12H22O11 (sugar) + H2O → C6H12O6 (glucose) + C6H12O6 (fructose)
Hydrolysis is the opposite of a condensation reaction. Condensation combines two molecules and releases water. Hydrolysis breaks one molecule into two smaller ones by adding water.[228]
Water can be broken down into hydrogen gas (H₂) and oxygen gas (O₂), using a process called electrolysis. In this process, an electric current is passed through water that contains small amounts of an electrolyte like sulfuric acid or salt. This helps the water conduct electricity. The electricity breaks down the water molecules:[229]
- 2H2O → 2H2 + O2
Electrolysis is important because it produces clean hydrogen fuel when powered by renewable energy like solar or wind. This is often called green hydrogen. It can be used to store energy, power fuel cells, or replace fossil fuels in industry and transportation.[230]
On Earth
[change | change source]Hydrology is the science that studies how water moves, where it is found, and its quality all over the Earth. It is part of a larger group of sciences that study water in different places and forms:
- Hydrography studies where water is located on Earth's surface.
- Hydrogeology focuses on groundwater, water stored underground.
- Glaciology studies ice and glaciers.
- Limnology looks at inland water bodies like lakes and rivers.
- Oceanography studies the oceans.
- Ecohydrology explores how water interacts with ecosystems and living things.
All the water on Earth (in oceans, rivers, lakes, ice, underground, and even in the air) is called the hydrosphere. The Earth holds about 1.386 billion cubic kilometers of water. Water on Earth is not distributed equally. Most of the water on Earth is found in the oceans. About 96.5% of all the water on Earth is salt water in the oceans. This cannot used for drinking or farming. That leaves only 2.5% of the Earth's water as fresh water. Of this small percentage of freshwater, most of them are in hard to reach places. Around 68.7% of Earth's fresh water is stored in ice caps, glaciers, and permanent snow. This is mainly in Antarctica and Greenland. Another 30.1% is found as groundwater, stored in aquifers deep under the Earth's surface. This groundwater is an important source of water for wells and springs. It is very important in agriculture and for drinking. Only about 1.2% of all fresh water is water that can be found on the surface of the Earth. This includes water in lakes (about 20.9%), swamps and marshes (2.6%), and rivers (0.49%). Water can also be found in the atmosphere as vapor, clouds, and precipitation, and in soil moisture, permafrost, and living organisms. But these make up only a tiny fraction of all the water on Earth (less than 0.01%).[19][20]
Fresh water is not evenly distributed across the Earth. Some places have more water than others. Countries like Canada, Brazil, and Russia have large supplies of fresh water because of lots of rivers and rainfall. In contrast, dry places like North Africa, the Middle East, and parts of Central Asia have very limited freshwater. Some tropical and temperate regions get lots of rainfall, while others may go months without much rain.[231]
Water cycle
[change | change source]
The water cycle (also called the hydrologic cycle) is the constant movement of water through the Earth's atmosphere and surface. Water moves between the air, ground, rivers, oceans, lakes, plants, and even underground. The main parts of the water cycle are:[232]
- Evaporation: Water from oceans, lakes, and rivers changes into vapor (gas) and rises into the air.
- Transpiration: Plants release water vapor into the air through their leaves.
- Condensation: Water vapor in the air turns into clouds.
- Precipitation: Water vapor in the air cools, turns into liquid or solid, and falls back to Earth as rain, snow, or hail.
- Runoff: Water flows across the land into rivers, lakes, and oceans.
The sun is the main source of energy that drives the water cycle. When sunlight heats up water, the water molecules move faster and can turn into vapor. This a process called evaporation. This happens in oceans, lakes, rivers, and even from the land. Plants also release water vapor through their leaves in a process called transpiration. Together, evaporation and transpiration are called evapotranspiration. The term evapotranspiration is commonly used by geologists. Water vapor is invisible to our eyes.[232]
Winds carry water vapor in the atmosphere over long distances. When water vapor cools down, it changes back into liquid water. This is called condensation. This can happen when warm air meets cool air. The water forms tiny drops around dust or salt called condensation nuclei in the air. These drops come together to form clouds. As more droplets stick together, they grow bigger. When they get heavy enough, they fall to Earth as rain, snow, or hail. This is called precipitation.[232]
When precipitation reaches the ground, it can do the following. It can evaporate again. It can flow over the land as runoff into rivers and lakes. Or soak into the ground to become groundwater. Water that flows in rivers and lakes is called surface water. Water that moves underground through soil and rock is called groundwater. Groundwater moves slowly and can come back to the surface through springs. It can also flow into rivers, lakes, or oceans. This shows how surface water and groundwater are connected.[232]
Most water that evaporates from the ocean goes back to the ocean. But wind blows water vapor to land at the same rate it goes back to the ocean. Each year, about 47 trillion tons of water vapor move from the ocean to the land. About 72 trillion more tons of water vapor comes from land evaporation and plants. That adds up to 119 trillion tons of precipitation falling on land each year.[233] Precipitation over land has many forms. Most commonly rain, snow, and hail, with some being fog and dew. Dew are small drops of water that form when warm, wet air touches a cool surface, usually in the early morning. Water droplets in the air may also refract sunlight to produce rainbows.[234]
Water that flows over land collects in places called watersheds. They then travel through rivers, carving out valleys and deltas. These areas usually have very good soil, which is good for farming and building cities. Sometimes, too much water causes a flood. This can happen when rivers overflow or big storms push water onto the land. Other times, there is not enough water. A drought happens when a place gets very little rain for a long time. This is often because of the place or shape of the land.
Water and Earth's geography
[change | change source]

Water plays an important role in shaping the Earth’s surface and what is happening under it. It helps form landscapes. It breaks down rocks. It even affects volcanic activity from deep under the surface. Water changes the land through both physical actions, like erosion, and chemical reactions, like breaking down minerals in rocks.
Water plays a big role in breaking down rocks on Earth’s surface. This is a process called weathering. There are two main types: physical and chemical. In physical weathering, water gets into cracks in rocks. When it freezes, it expands and makes the cracks bigger. Over time, this causes the rock to break apart. In chemical weathering, water mixes with carbon dioxide from the air or soil. This creates a weak acid called carbonic acid. Carbonic acid can dissolve some types of rock, especially limestone. When this acid gets to limestone underground, it can create large cracks, caves, and tunnels. On the surface, it can create a type of landscape called karst. Here the ground has sinkholes, caves, and holes. One amazing example of karst is the Stone Forest near Kunming, China. It has hundreds of tall, sharp towers of limestone made by water. Another example is Carlsbad Caverns in New Mexico, USA. This park has over 119 caves, all formed by water breaking down limestone. The biggest one, called the Big Room, is so large it could fit six football fields inside.[235][236]
Once rocks are broken down by weathering, the pieces are moved by a process called erosion. Water moves bits of rock and minerals away. No rock is strong enough to resist weathering and erosion forever. These powerful forces have created some of Earth’s most famous landmarks. For example, the Grand Canyon in Arizona, USA, was made by the Colorado River over millions of years. The canyon is about 446 kilometers (277 miles) long, up to 29 kilometers (18 miles) wide, and about 1.6 kilometers (1 mile) deep.[236]
Water is also very good at moving sediment. Sediment is made up of small pieces of rock, sand, and soil. Rivers, glaciers, rain, and even ocean currents move sediment over long distances. Eventually, this sediment settles in new places. Sediment is important because it adds nutrients to the soil. This helps plants grow. Places with lots of sediment, like riverbanks and deltas, are usually great for farming. They have a lot of different plants and animals. For thousands of years, the Nile River in Egypt flooded every year. This brought about 4 million metric tons of nutrient-rich sediment. Even today, the land next to the Nile is Egypt’s best farmland. Over millions of years, layers of sediment can press together to form sedimentary rocks. These rocks can contain fossils and clues about Earth’s past, like what the climate was like long ago.[237]
Deep inside the Earth, water plays an important role in forming magma. Magma is the hot, melted rock that can lead to volcanoes. The mantle is the thick layer under the Earth's crust. It is made of solid rock even though it is hot enough to melt rocks. This is because the pressure deep inside the Earth is so strong that it stops the rock from melting. But in places called subduction zones, one of Earth's tectonic plates goes under another plate. This brings water down into the mantle. This water lowers the melting point of the rock. This means the rock can start to melt even though it is still under very high pressures. This creates magma, which can rise and cause volcanic eruptions. So, water does not just shape the land on the surface, it also helps cause big changes deep underground. Over millions of years, water has helped shape how Earth looks and behaves.[238]
In the Universe
[change | change source]
Water is not only found on Earth. It can be found all over the universe. Astronomers have found ice, vapor, and sometimes liquid water in many places in the universe. Water ice has been found on the Moon, Mars, and comets. It has also been found on the icy moons of the outer planets, such as Europa and Enceladus. Even in interstellar space, the space between the stars, water exists as ice around tiny dust grains. It also exists as vapor in molecular clouds where new stars are born. Water has even been found in the atmospheres of exoplanets, planets outside our solar system.
A water molecule is made of two hydrogen atoms and one oxygen atom. Hydrogen came from the Big Bang. Oxygen was created inside big stars, much larger than the Sun. When these stars die and explode, they release oxygen into space. Oxygen can then combine with hydrogen to make water. Huge clouds of gas and dust, called stellar nurseries, are where new stars are born. These places often contain huge amounts of water vapor. For example, the Hubble Space Telescope found water molecules in the Helix Nebula. Water has also been found in young planetary systems around other stars. Around the star Beta Pictoris, which is about 20 million years old, scientists found water in a giant disk of gas and dust. This is likely from comets smashing into each other, asteroids, and forming planets. In the Orion Nebula, one of the biggest and most famous star-forming regions, water is still being made today. It is so large that it makes enough water every day to fill Earth’s oceans 60 times. All of this water, and the other molecules made in these star factories becomes part of new planets.[240] On 22 July 2011, scientists found a gigantic cloud of water vapor that had 140 trillion times more water than the Earth's oceans combined around a quasar 12 billion light years from Earth. According to the researchers, the discovery shows that water has been in the universe for a very long time.[241][242]
To find water in space, scientists make use of various tools and techniques. Telescopes with spectrometers can study the light coming from far away objects. Every molecule absorbs and emits light at specific wavelengths. By studying them, scientists can figure out whether there is water. Radio telescopes on the ground and space telescopes like the Hubble Space Telescope, the James Webb Space Telescope, and the Herschel Space Observatory have all helped us find water across the universe.[243][244][245][246][247] In our own solar system, spacecraft missions have also been very important. NASA’s Galileo and Cassini missions found strong evidence of subsurface oceans on Jupiter's and Saturn’s moons. Cassini found geysers on Enceladus, with water vapor and ice particles erupting from the moon’s surface.[248][249] On Mars, orbiters and rovers have found polar ice caps and certain rocks, which suggests that Mars might have had water in the past.[250][251]
In the solar system
[change | change source]


Water can be found in many places in our Solar System.[68][252] Earth is the only planet we know of that has liquid water on its surface all the time. Water can be found as: solid (ice), liquid (water), and gas (water vapor) on Earth. Scientists have found tiny amounts of water vapor in the Sun’s atmosphere. The Sun is very hot. Its surface is about 5,500°C (9,932°F) and the inside is even hotter. Normally, water breaks down into hydrogen and oxygen in such heat. But there are cooler spots on the Sun, like sunspots. At sunspots, temperatures can drop to about 3,000°C (5,432°F). That is still very hot, but cool enough for water molecules to form for a short time before they break apart into hydrogen and oxygen again.[253]
The Moon and Mercury both have water ice hidden in craters near their poles. These craters are always in the dark and never get sunlight. Because of this, the ice has stayed frozen for billions of years.[254][255] NASA spacecrafts, like the Lunar Reconnaissance Orbiter and MESSENGER, have found that these ice do exist.[256][257] Venus has water vapor in its atmosphere, just like Earth. But its surface is very hot and harsh. This means it does not have any liquid water on its surface. Scientists think Venus may have had water in the past, but it was lost into space. This is because Venus does not have a magnetic field to protect it like Earth does.[258] Mars has ice caps at its poles made of water and carbon dioxide. There is also ice under the ground in many places. Rovers and orbiters have also found hydrated minerals. This means that liquid water once flowed on the surface of Mars a long time ago.[68]
Asteroids are found near a part of the solar system called the “frost line”. This is the distance from the Sun where it is cold enough for water to freeze into ice. Beyond this line, you usually won’t find liquid water because it is very cold. It can only be found hidden under ice, mixed with salt that keep it from freezing, or trapped under pressure in an atmosphere. One example is Ceres, a dwarf planet. It may have a layer of dust and rock on the outside, with salty water ice deep under the surface.[259] Asteroids were once thought to be dry, but now scientists have found some that contain water ice or hydrated minerals. NASA’s OSIRIS-REx mission is helping us learn more about how much water some asteroids might have.[260][261] Comets are pieces of ice leftover from the early solar system. They have a lot of frozen water. When a comet gets close to the Sun, the ice turns into gas, creating the famous glowing tails.[262] There also exists a massive cloud of comets called the Oort cloud at the edge of the solar system.[68][263]
The outer planets, Jupiter, Saturn, Uranus, and Neptune also have water. They are big gas giants or ice giants. They do not have a solid surface to walk on like our planet. Jupiter has water vapor in its thick atmosphere, but it is hard to see because of all the clouds.[264] Saturn is like Jupiter. It has some water vapor in its atmosphere. Its beautiful rings are mostly made of water ice.[265] Uranus might have an icy layer deep under its atmosphere.[266] Neptune is similar to Uranus. Scientists think it also has an icy layer under its atmosphere that may contain water and other ices.[68][267]
Some of the moons around the giant planets in our Solar System may have huge oceans of liquid water under their frozen surfaces. Europa, Ganymede, and Callisto, which orbit Jupiter, and Enceladus, and Titan, which orbit Saturn, might have underground oceans. Enceladus, a small moon of Saturn, shoots out jets of water vapor and ice from its south pole. A spacecraft named Cassini flew through these jets and found water, salts, and simple organic molecules. These are clues that there might be a hidden ocean with life. Titan, Saturn’s biggest moon, has lakes and seas on its surface, but they are filled with liquid methane and ethane, not water. Scientists think Titan has a salty water ocean deep underground, hidden under its icy surface. The moons of Uranus also have icy surfaces. Titania, the largest, has water ice and carbon dioxide ice. There might be liquid water under its surface. Triton, Neptune’s biggest moon, has a surface of frozen water ice. Deep under that ice, scientists also think it might have a liquid ocean.[68][268]
The dwarf planet Pluto has a surface covered in frozen nitrogen and water ice. Pluto is extremely cold and far from the Sun. Scientists think Pluto might have a liquid ocean deep beneath its icy crust, about 100 kilometers deep.[269] Pluto’s biggest moon, Charon, also has water ice. Beneath the surface, Charon may have had liquid water in the past. Some scientists think ice geysers might still happen on Charon.[68]
In interstellar space
[change | change source]
Water is not only found on planets and moons. It can also be found in the vast spaces between stars, the interstellar medium. The interstellar medium (ISM), is the scattered mix of gas, dust and radiation that can be found in the space between the stars in a galaxy. Water can be mainly found as ice covering dust grains in the interstellar medium and dense molecular clouds, where new stars are born. It can also be found as water vapor inside dense molecular clouds. In the coldest parts of the ISM, water molecules condense onto the surfaces of dust grains, forming icy mantles. These icy grains are very important ingredients in the chemistry of making stars and planets.[270][271][272]
Molecular clouds, places where stars and planets are born, contain huge amounts of water. Water vapor has been found in many of these clouds, especially near newly forming stars. As young stars fuse hydrogen into helium they release heat around them. This warms up the surrounding ices, turning them into water vapor. This allows them to be observed by space telescopes such as the Herschel Space Observatory and the Spitzer Space Telescope.[273][274] One of the most famous places where water has been studied is the Orion Nebula. Here, astronomers have found huge amounts of water vapor surrounding stars being born.[275][276] In these environments, water is also very important in the chemistry that creates more complex organic molecules like amino acids.[277][64] The Rho Ophiuchi cloud complex is a nearby molecular cloud about 460 light-years away. It contains protostars surrounded by water-rich ices. The ice in this region has been found using telescopes like Spitzer and JWST.[278][279][280]
Ice and tiny grains of dust were the main ingredients that came together to form the Solar System. Scientists believe that the water in the solar system formed in space before our Sun or planets even existed. When star systems begin to form, gravity pulls together this gas and dust to make stars and planets. The dust already has water on it, which can become part of the new planets. That means planets like Earth might have been born with water already inside them.[281]
On exoplanets
[change | change source]
When looking for life on other planets, called exoplanets, water is one of the most important molecules scientists look for on other planets. We cannot use a telescope to look into other planets for water because they are very far away. But, astronomers have found others ways to look for water on some of these far away planets. The main way scientists study planets outside our Solar System is through something called transit spectroscopy. This means watching a planet as it passes in front of its star. When it does, some of the star’s light goes through the planet’s atmosphere to us. Depending on what the atmosphere is made of, certain parts of the light get blocked or bent. This creates a kind of “fingerprint” in the light that scientists can study. By looking closely, they can figure out which gases are in the atmosphere, like water or methane. Right now, studying the atmospheres of exoplanets is still very hard. Our tools are not perfect for this yet and it takes very careful measurements.[282]
This method has found water vapor on many "hot Jupiters", such as HD 189733 b and HD 209458 b. These are large gas giants very close to their stars. Though these planets are far too hot for liquid water. Water exists as water vapor in the atmospheres of these planets.[283][284] In recent years, telescopes have begun finding signs of water on smaller planets, especially sub-Neptunes and super-Earths. These are planets smaller than gas giants but larger than the Earth. One example is K2-18b, a super-Earth found by NASA's Kepler spacecraft in 2015. It is a planet with eight times the mass of the Earth that orbits a so called red dwarf star, which is much cooler than the sun. K2-18b can be found in the “habitable zone” of its star. This means it has the right temperature to have liquid water. Given its mass and radius, K2-18 b may either be a rocky planet with a thick atmosphere or be more like Neptune. Observations with Hubble and the James Webb Space Telescope (JWST) have found water vapor in its atmosphere. Though scientists cannot say if it has clouds, oceans, or even rain. Planets in the habitable zone, are places scientists are looking with new technology like the James Webb Space Telescope.[285]
The search for extraterrestrial life
[change | change source]When scientists look for life in space, they often start by looking for water. Every living thing on Earth needs water to survive. Water has special chemical properties that make it perfect for life. It is really good at dissolving other substances, which helps living things carry out chemical reactions. Water stays liquid over a wide range of temperatures. It can help keep living organisms from getting too hot or cold. It also helps move nutrients and waste in and out of cells and living organisms. That is why scientists often say “follow the water” when searching for life beyond Earth.[286]
Scientists think moons like Europa (which orbits Jupiter) and Enceladus (which orbits Saturn) might be able to have life. Both moons have thick layers of ice on their surface. Under that ice, scientists believe there are massive oceans of liquid water. The bottom of these oceans could be like hydrothermal environments found in the deep sea on Earth. On our planet, hydrothermal vents release hot, mineral-rich water from under the seabed. This creates a place where life thrives without sunlight. Instead of using sunlight for energy (like plants do), the organisms living there use chemical energy from the minerals in the water. This is a process called chemosynthesis. If similar hydrothermal vents exist on Europa or Enceladus, they could provide the energy and nutrients needed for life to survive in complete darkness, just like Earth’s deep sea life.[287][288] Scientists have already found clues supporting these ideas. On Enceladus, for example, NASA’s Cassini spacecraft found jets of water vapor, ice particles, and organic molecules shooting out from cracks from the moon. This is a strong sign that there is liquid water and hydrothermal vents under its icy surface. These discoveries make Europa and Enceladus key targets for future space missions.[289]
Mars remains a key target in the search for life beyond Earth. Although the planet is cold and dry today, it shows strong signs that it might have had water in the past. Ancient river valleys, dry lake beds, and minerals that only form in water suggest that liquid water once flowed on the Martian surface. Scientists believe that billions of years ago, Mars may have had a thicker atmosphere and a milder climate, possibly making it a habitable world. Even now, there may be salty liquid water hidden underground, especially near the poles or beneath the surface. Briny water, which contains dissolved salts, can stay liquid at colder temperatures, increasing the chances that it might still exist on Mars. To explore this possibility, NASA and other space agencies have sent robotic missions like the Perseverance and Curiosity rovers to study Mars up close. These rovers have advanced tools to analyze rocks, soil, and even the atmosphere. They are looking for biosignatures, which are chemical or physical signs that could suggest past life.[290][291][292]
Scientists often look for water when they search for life on exoplanets. And they often look for planets in the habitable zone. This is the area around a star where temperatures are just right for liquid water to exist on a planet. If a planet is too close to its star, it will be too hot and water will boil away. If it is too far, it will be too cold and water will freeze. But in the habitable zone, things can be just right for water to stay liquid. Earth can be found in the habitable zone of the Sun. This is one reason it has oceans, rivers, and rain. That is why it can support so many kinds of life. When scientists study planets around other stars (called exoplanets), they look to see if those planets are also in the habitable zone. If they are, and they have the right kind of atmosphere, they might have water. And, they might even have life. Beyond our solar system, astronomers have discovered water vapor in the atmospheres of some exoplanets. These findings suggest that water, a key ingredient for life as we know it, might be common in the universe. This ongoing research brings us closer to answering one of the biggest questions in science: Are we alone in the universe?[293][294]
Uses of water
[change | change source]Plants and animals (including people) are mostly water inside, and must drink water to live. It gives a medium for chemical reactions to take place, and is the main part of blood. It keeps the body temperature the same by sweating from the skin. Water helps blood carry nutrients from the stomach to all parts of the body to keep the body alive. Water also helps the blood carry oxygen from the lungs to the body. Saliva, which helps animals and people digest food, is mostly water. Water helps make urine. Urine helps remove bad chemicals from the body. The human body is between 60% and 70% water, but this value differs with age; i.e. a foetus is 95% water inside.
Water is the main component of drinks like milk, juice, and wine. Each type of drink also has other things that add flavor or nutrients, things like sugar, fruit, and sometimes alcohol. Water that a person can drink is called "potable water" (or "drinking water"). The water in oceans is salt water, but lakes and rivers usually have unsalted water. Only about 3% of all the water on earth is fresh water. The rest is salt water.[295][296]

Many places, including cities and deserts, don't have as much water as people want. They build aqueducts to bring water there.
Though people can survive a few months without food, they can only survive for a day or two without water. A few desert animals can get enough water from their food, but the others must drink. Water has no smell, taste, or color.
Water is also used for recreational purposes, see list of water sports.
Water is used as both the coolant and the neutron moderator in most nuclear reactors. This may be ordinary water (called light water in the nuclear industry) or heavy water.
Water is also used for washing a lot of objects. Goods, services and people are transported to other countries in watercrafts on bodies of water.
Water is used in chemical reactions as a solvent or reactant. Water is also used in fire fighting. Water is also used for cooking.
Water laws, crisis, and geopolitics
[change | change source]Water laws
[change | change source]
Water laws are rules that help decide how water is shared, used, and protected. These laws are important because water is something that everyone needs. People, farms, businesses, and nature all depend on water. Water laws help figure out who can use water, how much they can use, and what they can use it for. They also try to make sure that water is used fairly and not wasted or polluted. In many places, water is limited, and if it is not managed well, it can run out or get too dirty to use. Water laws are meant to stop problems like this including fights over water. They also help make sure there is enough clean water for things like drinking, growing food, making energy, and protecting the environment.[297]
Water rights are the rules about who can use water and how much they can use. These rules are part of water laws.[298] There are different kinds of water rights depending on where you live and the laws people follow. One common system is called riparian rights, which is often used in places with plenty of water. With riparian rights, if you own land next to a river, lake, or stream, you have the right to use the water. But you have to be careful not to harm the people who live downstream.[299] In drier places, they often use a different system called prior appropriation. This follows the rule of "first in time, first in right." The first person to take water for a useful purpose has the right to keep using it, even if their land is not next to the water.[300] In many parts of the world, especially in rural or indigenous communities, customary or traditional laws also guide how water is shared. These laws are based on old practices and cultural values that help people respect and protect water.[301] Different countries have their own laws and rules for managing water. Some countries focus on deciding who can use water and how they can use it, while others pay more attention to stopping pollution or building things like dams and pipelines to help control water. When rivers, lakes, or underground water cross borders between countries, water law becomes more complicated. In these cases, countries need to work together and make agreements to share the water fairly and avoid fights.[302] For example, many countries share big rivers like the Nile, Mekong, and Danube. These countries cooperate through special agreements to manage the water in a way that works for everyone.[303]
Water use is usually managed by national or local governments and special government agencies. These groups decide how much water people can use, check that the water is clean and safe, and protect the plants and animals that need water to live. They also help solve problems when two communities or businesses want to use the same water source. Water governance means making sure water systems are cared for, pollution is controlled. It also means making sure everyone has access to clean water for drinking and hygiene. Good water management is very important to handle problems like droughts, floods, growing populations, and climate change.[304] There are many important examples of water laws and how they affect people. In the United States, the Clean Water Act is a big law that helps protect rivers, lakes, and other water from pollution.[305] In Africa, the countries along the Nile River have talked and sometimes argued for a long time about how to share the river’s water fairly.[306] Some countries are starting to say that water is a public good or even a human right. This means that everyone should be able to get clean water, no matter how much money they have or who they are.[307]
Water crisis
[change | change source]

A water crisis is a serious situation that happens when people do not have enough clean and safe water to meet their daily needs for drinking, cooking, washing, and farming. It also includes times when water is available but too polluted or too far away to be used safely. A water crisis can also affect the environment. It can harm rivers, lakes, and plants and animals that depend on water. This problem is not just about having no water at all. It also includes having water that is too dirty, too expensive, or too difficult to reach. There are many reasons why water crises happen.[308]
One major cause is population growth. As more people are born and the world’s population grows, more clean water is needed for drinking, cooking, washing, and growing food. As cities get bigger and more people use water, rivers, lakes, and underground water sources are being used up faster than nature can replace it. This causes a water scarcity, which means there is not enough clean water for everyone’s needs. In big cities, the problem can get worse. When lots of people live close together, they all need water at the same time. In poor neighborhoods or fast-growing areas, some people may not have any clean running water at all. In the countryside, farmers use a lot of water to grow crops and feed animals. As the population grows, the demand for food grows too, so farmers need even more water. Some use strong pumps to pull water from underground, but if they take too much, the water underground can run out.[309][310][311]
Another big problem is pollution. Even though there is water in rivers, lakes, and oceans, a lot of it is not safe to drink or use because it is polluted. Pollution happens when harmful things like chemicals, trash, or dirty water from homes and factories get into natural water sources. This makes the water dangerous for people, animals, and plants. One major source of pollution is waste from homes and cities. When people wash dishes, take showers, or flush toilets, that dirty water has to go somewhere. In some cities, it goes to special treatment plants that clean the water. But in many places around the world, there are no good systems, so the dirty water goes straight into rivers or lakes. This can carry germs that cause serious diseases like cholera or diarrhea. Even in big cities, if the water system breaks or gets too full during a storm, dirty water can mix with clean water and make it unsafe to drink. Factories and farms also cause a lot of water pollution. Some factories dump leftover chemicals, oil, or heavy metals into nearby rivers to save money. These dangerous substances poison the water and can build up in fish, making them unsafe to eat. On farms, people use fertilizers and pesticides to help crops grow and kill bugs. But when it rains, these chemicals can wash off the land into rivers or lakes. This is called “water runoff.” It can make algae grow too fast. When the algae die, they use up the oxygen in the water. Then fish and other animals cannot survive, and parts of the water become “dead zones” where nothing can live. Plastic is another big problem. Every year, tons of plastic bottles, bags, and wrappers end up in rivers and oceans. Plastic does not break down easily, so it can float in the water for years. Sea animals like turtles or fish might eat plastic by mistake and die. Tiny pieces of plastic, called microplastics, have even been found in drinking water and seafood. Scientists are still studying how these microplastics might affect human health.[312][313][314][315]
Another big reason for water problems is overuse. This means people are using too much water too fast, much faster than nature can refill it. Every day, water is needed for many things like drinking, cooking, cleaning, farming, making clothes, building houses, and running factories. As more people live on Earth, they use more water. But there is only a limited amount of fresh water on the planet. When we take too much water without giving nature enough time to replace it, rivers dry up, lakes get smaller, and underground water sources called aquifers drop lower and lower. Farming uses the most water of all. Farmers need water to grow crops and take care of animals, but in many places, they use more water than necessary. Sometimes they flood their fields, and a lot of the water is wasted or lost to evaporation. In dry places, farmers often pump water from deep underground. At first, this works well, but if they keep pumping too much, those underground water supplies start to run out. Once an aquifer is empty, it can take many years or even centuries to fill up again. This is happening in parts of the United States, India, China, and many other countries. Factories and industries also use huge amounts of water to make things like paper, steel, clothes, and electronics. For example, it takes thousands of liters of water to make just one pair of jeans or a hamburger.[316][317][318]
Another big reason for the water crisis is poor infrastructure. Infrastructure means the systems and buildings we use to carry, store, clean, and deliver water, like pipes, pumps, water treatment plants, and storage tanks. In many places, especially in poorer countries or old cities, these systems are old, broken, or not built well. This means that even if there is enough water nearby, people still cannot get clean, safe water in their homes. Because of this, millions of people either get no water at all or have to use water that can make them sick. In some poor or rural areas, there might be no water system at all, so people have to walk long distances to rivers or wells to get water by hand. Sometimes this water is not clean because there is no way to filter or clean it. In cities, even when pipes and pumps exist, they may be old and not well cared for. Leaking pipes waste a lot of water, sometimes up to half of it is lost before it even gets to homes or schools.[319] In crowded neighborhoods, especially slums, water pipes may only work a few hours a day or not at all. People there have to buy water from trucks or use unsafe sources. Water treatment plants are supposed to clean the water by removing dirt, germs, and chemicals before people drink it. But if these plants are too small, do not have enough money, or are broken, the water from taps can still be unsafe. Dirty water can cause diseases like cholera, typhoid, and diarrhea, which kill thousands of people every year, especially children. Sometimes, clean water and dirty sewage use the same underground pipes. When those pipes break or flood, the two can mix, making people sick without them knowing. As more people move to cities, they need more water, but water systems do not always grow fast enough to keep up. During heat waves or droughts, the systems can break down even more. And during floods, water pipes may burst or get clogged, leaving people without water for days.
Another big reason the world has a water crisis is that governments often do not manage water well. Even if a country has enough water, bad planning, weak laws, and unfair choices can cause big problems. In many places, water is not shared fairly. Some people, especially those in rich neighborhoods or powerful companies, get plenty of clean water, while others in poor areas get very little or none at all. Sometimes governments build big water projects like dams or canals, but these only help a few people and leave out whole communities. Poor water planning also causes waste and shortages. In some countries, water is used without limits. Big farms or factories take as much water as they want from rivers or underground, and the government does not keep track of it. This causes overuse, where water is taken faster than nature can replace it. Also, water bills may be too cheap or not collected at all, so people do not try to save water. In other places, governments do not spend enough money on water systems, so pipes leak, treatment plants break, and many areas have no working water services. Sometimes, powerful people or companies bribe officials to get more water than they need, while others are ignored. Governments may promise to build water projects but never finish them, or the money disappears because of corruption. Sometimes leaders make decisions without asking local people what they really need, so water projects do not fix real problems. For example, a city might build a big water pipeline but forget to build toilets or drains, which can cause health problems. Another issue is that many governments do not plan for the future. As cities grow and the climate changes, water needs change too. But without good planning, old water systems cannot keep up. When droughts happen, there are no backup plans. When floods come, water systems break down.
At the same time, climate change is making the water problem worse. As the Earth gets hotter, it changes how water moves in nature. Normally, water falls as rain or snow, flows into rivers and lakes, and then evaporates back into the air. But with climate change, this natural water cycle gets out of balance. In many places, rain does not come when it is needed, or it falls all at once in heavy storms that cause floods. Some areas are getting drier, while others get too much water. This makes it harder for people to get clean and steady water when they really need it. In dry places, climate change causes droughts to happen more often and last longer. A drought is when there is very little rain for a long time. This causes rivers to shrink and lakes to dry up. Crops would not grow, and animals would not have enough water to drink. Farmers suffer the most because they need water to grow food and take care of their families. On the other hand, some places get too much water at once. Heavy rain from warmer temperatures can cause floods that destroy homes, farms, and water systems. Flood water can mix with sewage and chemicals, making it dirty and unsafe to drink. Even though floods bring lots of water, it is often not clean or useful. Also, glaciers and snowpacks in mountains are melting faster than before. These usually act like natural water tanks, slowly releasing water during dry months. But now, they are disappearing, which means millions of people who live downstream will have less water in the future.
The world is facing a very serious water crisis, and the numbers show how big the problem really is. Right now, more than 2.2 billion people, almost 1 out of every 4 people on Earth, do not have safe drinking water at home. That means they cannot just turn on a tap to get clean water to drink, cook, or wash with. Even more people, about 3.5 billion, do not have proper sanitation, like toilets or safe ways to get rid of dirty water. Without clean water and safe toilets, diseases spread easily, and many children, especially in poor areas, get sick. According to the World Health Organization, around 500,000 people die every year from diseases caused by dirty water and poor hygiene. About 4 billion people, more than half the world’s population, face serious water shortages for at least one month every year. Some of the countries with the worst water problems include India, Egypt, Iran, and parts of the United States, like California. In these places, rivers are drying up, underground water is running low, and lakes are shrinking. For example, the Aral Sea, which used to be one of the largest lakes in the world, has almost completely dried up because people used too much of its water. In Cape Town, South Africa, the city almost ran out of water in 2018. The crisis was so bad they called it “Day Zero,” the day the taps would be turned off if water ran out. Climate change is making things worse. As the planet gets hotter, droughts are happening more often and lasting longer. Rain patterns are changing, so some places get too much rain all at once, causing floods, while other places don’t get enough rain, leading to dry land and crop failures. The United Nations says that by 2050, over 4 billion people might not have safe water at home unless we make big changes. Even in places that have enough water, the systems that deliver it do not always work well. The World Bank says the world loses about $260 billion every year because of bad water systems, like leaking pipes, poor planning, or people getting sick and missing work or school because of waterborne diseases. At the same time, people living in slums or rural areas often have to pay more money per liter for water than rich people in cities. That is because they have to buy it from private sellers or carry it from far away. This is unfair and keeps many families stuck in poverty.
There are many ways we can fix the water crisis, but it takes everyone working together, including governments, businesses, communities, and ordinary people. One of the most important steps is to build and fix water systems. This means making sure pipes, pumps, treatment plants, and tanks work well and bring clean water to everyone who needs it. In many poor places, there are no water systems at all, so people have to walk far to get water. By investing in strong water systems, clean water can be delivered straight to homes, schools, and hospitals. Good systems also stop leaks, so less water is wasted before it even reaches people. Another important solution is to use water more carefully and waste less. Many people and factories use more water than they really need. Simple things like fixing dripping taps, taking shorter showers, and turning off the water while brushing your teeth can save a lot of water. Farmers, who use most of the world’s water, can use better methods like drip irrigation and collecting rainwater to grow crops with less water. In cities, recycling water from sinks and showers to flush toilets or water plants helps save even more. Protecting and cleaning our water sources is also very important. Rivers, lakes, and underground water can get polluted by trash, chemicals, and sewage. To keep water clean, we need to stop dumping waste into nature. Governments should make strong laws to control pollution from factories and farms. People can help too by not throwing garbage into rivers or drains, and by using less plastic and fewer harmful cleaning products. Fair government rules are needed as well. In some places, water is not shared fairly. Rich neighborhoods and big companies get plenty of water, while poor communities get very little. Governments must treat water as a basic human right, not just something to buy and sell. They should plan for the future by knowing how much water is available, who needs it most, and how to protect it from climate change. They should also include local people when making decisions about water use and sharing. Education is another important part of solving the water crisis. Many people do not know how serious the problem is or what they can do to help. Schools, TV shows, and community projects can teach kids and adults about water and why it’s important to save it. Finally, technology can help a lot. New tools like water filters, low-water toilets, smart irrigation systems, and apps that track water use make saving water easier. Even simple tools like tanks that collect rainwater from rooftops can give families clean water in dry areas. Scientists and engineers are working on ways to clean salty ocean water, safely recycle wastewater, and watch water use from space. All these ideas together can help make sure there is enough clean water for everyone.
Water geopolitics
[change | change source]
Water is one of the most important resources on Earth. People need it to drink, cook, clean, and grow food. But when there is not enough water, or when it is not shared fairly, it can lead to conflict between people, groups, or even countries. One major cause of water conflict is scarcity. Scarcity means there is not enough water for everyone who needs it. This can happen in dry places where it does not rain much, or in areas where the population is growing quickly and using too much water. When water is limited, people may start to argue or fight over who gets to use it. For example, if two farmers both use the same river to water their crops, but the river starts to dry up, they may blame each other and argue about who should get more water. These kinds of problems can happen between neighborhoods, cities, or even whole countries that share rivers or lakes.
Another reason people fight over water is when rivers or lakes cross borders between countries. These are called transboundary water sources, and they can cause serious problems. For example, the Nile River flows through several countries in Africa. Each country wants to use the water for things like farming and electricity. If one country builds a dam or takes more water than before, the others may get upset or worried they would not have enough. The same kind of conflict happens in other parts of the world. India and Pakistan have had disagreements over the Indus River, and countries in Central Asia have argued over rivers that come from melting mountain glaciers. If countries do not find fair ways to share the water, it can lead to political fights and even war. Pollution is another cause of water conflict. When factories, farms, or cities dump waste into rivers or lakes, the water becomes dirty and unsafe. If one group pollutes the water and another group depends on it for drinking or farming, big problems can happen. People downstream may get sick or lose their crops and blame those upstream. This can lead to lawsuits, protests, or even violence. Even within one country, different areas or groups might argue about who is polluting the water and who should pay to clean it up. These fights can make it even harder to solve the water crisis.
Unfair sharing of water can also cause problems. In many places, rich neighborhoods or big companies get more water than poor communities. When there is not enough water for everyone, and some people have plenty while others have none, it can make people upset and angry. This can lead to protests or even fights. For example, in some cities, poor areas called slums may have no running water at all. But at the same time, fancy hotels nearby might use a lot of water to fill swimming pools or run fountains. When people see this kind of unfairness, they may feel they are being treated badly and demand change. Climate change is also making water conflicts worse. As the Earth gets hotter and rain becomes less regular, droughts are happening more often. This puts more stress on water supplies and makes people compete even more. In some places, climate change is melting glaciers and drying up rivers. When water becomes harder to find, people worry more and fight harder over the little that is left. If governments do not step in to share water fairly and plan for the future, these conflicts could become more serious over time.
One of the most well-known water conflicts in the world is happening along the Nile River in Africa. The Nile is the longest river in the world, and it flows through 11 different countries, including Ethiopia, Sudan, South Sudan, Egypt, and Uganda. This river is very important because millions of people use it every day for drinking water, farming, fishing, and making electricity. But since so many countries share the Nile, there are many disagreements about how the water should be divided. The biggest conflict is between Ethiopia, Sudan, and Egypt. Each country depends on the Nile for different reasons, and they do not always agree on what is fair. A major reason for the conflict is a huge dam that Ethiopia is building. It is called the Grand Ethiopian Renaissance Dam, or GERD. Ethiopia started building this dam in 2011 on the Blue Nile, one of the two main branches of the Nile River. Ethiopia says the dam is important because it will create electricity for millions of people who do not have enough power. They believe it will help the country grow and develop. However, Egypt is very worried about the dam. Egypt gets about 90% of its water from the Nile, and it fears the dam will lower the amount of water flowing to its farms and cities. Egypt needs the Nile for almost everything, especially to grow crops in the desert. Sudan is in the middle. On one hand, the dam could help Sudan by reducing floods and giving them more electricity. On the other hand, Sudan is worried that Ethiopia might fill the dam too quickly, which could reduce the amount of water Sudan receives for farming and drinking. Sudan wants all three countries to make a clear agreement so that everyone can benefit and no one gets harmed. Ethiopia, Egypt, and Sudan have been talking about this issue for many years, but they still have not made a final deal. Egypt wants strict rules about how and when Ethiopia can fill the dam, especially during dry years. Ethiopia wants to control the dam itself and believes it has the right to use the river that starts in its land. Because of these disagreements, there have been many arguments, tense meetings, and even threats. Some people worry that if the countries cannot work together, it could lead to a serious crisis or even conflict.
Another example of water conflict is between India and Pakistan. These two countries have not always gotten along, and sharing water has made things even more difficult. The Indus River and its smaller rivers start in India but flow into Pakistan. In 1960, the two countries signed a water treaty to divide the rivers fairly. But even today, each country is worried that the other might take more water than they should. When India builds new dams or water projects, Pakistan gets alarmed and accuses India of breaking the agreement. These water issues add to the already tense relationship between the two countries. In the Middle East, water is very limited, and this leads to many conflicts. One example is the Jordan River, which flows through Israel, Jordan, and Palestine. All three groups need the river for drinking water, farming, and everyday life. But over the years, the river has shrunk because of overuse and pollution. As the water supply drops, arguments have grown. In the West Bank, many Palestinian communities struggle to get enough water, while nearby Israeli settlements often have steady supplies. This unfair access makes people angry and adds to the political tension. Some efforts have been made to share the water more fairly, but it is hard because the groups do not trust each other. In Yemen, a country dealing with war and poverty, water shortages have also led to conflict. Many people there do not have clean water. As wells dry up, villages and tribes sometimes fight over who gets to use the last water sources. In some rural areas, armed groups have taken control of water supplies, making it even harder for families to get the water they need. Some experts believe that water problems have made Yemen’s civil war even worse. This shows how lack of water can become even more dangerous when a country is already in crisis.
In the United States, water conflicts happen more often than people might think, especially in the western part of the country where water is harder to find. One of the biggest and most well-known water fights is over the Colorado River. This river flows through seven states: Colorado, Wyoming, Utah, New Mexico, Arizona, Nevada, and California. It also flows into Mexico. More than 40 million people use the Colorado River for drinking water, farming, and making electricity. But lately, the river has been shrinking because of drought, climate change, and using too much water. The states argue over how much water each one should get. For example, California uses a lot of water for growing food in the desert, while big cities like Las Vegas and Phoenix need more water as more people move there. The government has tried to help the states make fair deals, but it’s been very hard to agree on a long-term solution. Another big water conflict in the U.S. is between Georgia, Alabama, and Florida. This fight has been going on for decades. These states share rivers like the Chattahoochee and the Flint, which come together to form the Apalachicola River. Georgia wants more water for the city of Atlanta and for farms. But Alabama and Florida say that if Georgia takes too much water, there would not be enough left for them. Florida especially needs the water to keep its seafood industry alive in Apalachicola Bay. This fight has gone all the way to the U.S. Supreme Court, but even after many years, it is still not solved. Even in places with more water, like around the Great Lakes, there can still be conflicts. Some towns and companies want to take more water out of the lakes for their own use, but others worry this could hurt the environment or lower the water levels. The Great Lakes are shared by several U.S. states and Canada, so any plan to use the water has to be agreed on by everyone. In 2008, a deal called the Great Lakes Compact was made to protect the lakes and stop people from taking water unfairly. Still, some places try to find ways around the rules, which causes more arguments and concern.
Even though water can sometimes cause conflict, it can also help bring people together. Many countries, states, and communities have worked hard to cooperate and make fair deals about how to share water. These water agreements are very important because they help prevent arguments, protect nature, and make sure everyone has the water they need. When countries share rivers, lakes, or underground water, they often create treaties, or written agreements. These treaties explain how much water each side can use and what to do if there is a problem. They might also include plans to keep the water clean and to stop pollution and waste. One famous example is the Indus Waters Treaty between India and Pakistan. This treaty was signed in 1960 with help from the World Bank. The Indus River system flows through both countries and is very important for farming and daily life. Even though India and Pakistan have had many political problems and even wars, this water treaty has mostly worked well. It divides the rivers between the two countries and includes rules about building dams and using the water fairly. The treaty has helped stop big water fights for over 60 years. This shows that even countries that do not get along can work together when it comes to water.
Another important example of water cooperation is the Senegal River Basin agreement in West Africa. The Senegal River flows through several countries, including Senegal, Mali, and Mauritania. In 1972, these countries created a group called the Organisation for the Development of the Senegal River (OMVS). They agreed to manage the river together, share the water fairly, and build dams and power plants that help all the countries. The group listens to farmers, fishermen, and local leaders to make sure everyone’s needs are heard. This kind of teamwork helps people trust each other and solve problems peacefully. In Europe, there are many rivers that cross between countries. One of the most important is the Danube River, which flows through more than 10 countries. In 1994, these countries signed the Danube River Protection Convention. They promised to work together to keep the river clean, prevent floods, and protect the plants and animals that live in and around the water. The agreement includes regular meetings, water testing, and teamwork during emergencies. Because of this, the Danube remains safe and helpful for millions of people. The United Nations (UN) also supports water cooperation around the world. In 1997, the UN created a special agreement called the Convention on the Law of the Non-Navigational Uses of International Watercourses. This set of rules encourages countries to share water fairly, avoid harming each other, and settle arguments peacefully. Not all countries have signed it, but it still helps guide how nations work together to manage shared rivers and lakes. The UN also celebrates World Water Day every year on March 22 to remind everyone how important it is to work together on water issues.
In culture
[change | change source]In religion
[change | change source]
Water has deep meaning in many cultures and religions around the world. Almost everywhere, water is seen as a symbol of purity, life, and change. It often stands for cleansing, not just of the body but also of the spirit. People believe water can wash away bad things and help start fresh. It is important to many religious beliefs and spiritual practices. It can represent the cycle of life, rebirth, and the connection between the physical world and something sacred or divine. In many faiths, water is used in religious rituals to bless or purify people. These ceremonies include baptisms, ritual washings, and blessings. For example, people may wash their hands, face, or whole body before prayer or entering a sacred place to show respect and prepare for spiritual contact. In baptism, a person is dipped in or sprinkled with water to mark the start of a spiritual journey. It often means the washing away of sins and beginning a new life. Water may also be sprinkled or offered during other ceremonies to bless people, objects, or spaces. These practices show the belief that water has spiritual power.[320][321]
Different religions use water in special ways. In Christianity, baptism is an important ceremony where water is used to show spiritual cleansing and a new life in Jesus Christ.[322] In Islam, water is used in wudu (a small washing) and ghusl (a full-body washing). These are done before prayers and other holy activities to be clean in body and spirit.[323][324] In Hinduism, water is very sacred, especially rivers like the Ganges. People believe this river can wash away sins. It is used in many ceremonies, including funerals.[325][326] Buddhists use water in temple rituals as a sign of offering and for spiritual cleansing.[327] In Judaism, there is a special bath called a mikveh. People use it for purification during important times, like before getting married or when entering the religion.[328] In Sikhism, being clean is part of being spiritual. Sikhs use water for bathing and in some religious rituals.[329] Many indigenous and animist traditions also see water as sacred. Rivers, lakes, and springs are believed to be the homes of spirits or gods. They are often part of rituals and traditional stories.[330]
Sacred water sources are very important in many religions. People often travel long distances to visit these special places during pilgrimages. These water sites are believed to have healing powers or a spiritual presence.[331] For example, in Christianity, many people visit the spring at Lourdes in France, which is believed to heal the sick. Every year, millions of people go there to pray and collect water.[332] In Hinduism, the Ganges River is one of the most holy rivers. Bathing in it is believed to wash away sins and bring blessings.[326] In Islam, the Zamzam Well in Mecca is a sacred part of the Hajj pilgrimage. It has deep spiritual and historical meaning for Muslims.[333]
In philosophy
[change | change source]
In ancient times, people believed that everything in the world was made from a few basic things called elements. One of the first known Western philosophers, Thales of Miletus, lived in the 6th century BCE and thought that water was the most important of them all. Thales believed that water was the origin of everything. He called it the "archê," a Greek word that means "first principle" or "beginning." He noticed that water is essential for life and can exist in three forms: solid, liquid, and gas. Because of this, he thought water was the basic building block of the universe. Thales was one of the first people to try to understand the world through observation and reason, instead of relying on myths or stories. His ideas helped start what we now call natural philosophy, the early form of science.[131]
As philosophy grew in different parts of the world, many cultures gave water an important place in their way of thinking. In ancient Greece, famous philosophers like Plato and Aristotle believed that everything was made of four main elements: earth, air, fire, and water. Each element had special qualities. Water was thought to be cold and wet. It was linked to change and movement because of the way it flows.[334] In Eastern philosophy, water also had deep meaning. In Daoism, a major tradition from China, water is a symbol of ideal behavior. It flows gently, adapts to its surroundings, and can slowly wear down even hard rock, not by force, but with patience and persistence.[335] This idea is part of the Daoist teaching called wu wei, which means acting without effort or force.[336] In Indian philosophy, water is one of the Pancha Mahabhuta, or five great elements, in both Hinduism and Buddhism. Water stands for emotion, cleansing, and flow. It is seen as important not just for the body, but also for the soul and the balance of nature.[337]
Throughout history, philosophers have used water as a symbol or metaphor in their writing. Water is often used to represent the flow of time, the way we think and feel, or how things can change shape while staying as the same substance. In many traditions, water stands for change or transformation. It can turn into ice or steam, then go back to being liquid again. Because of this, philosophers use water to talk about how life is always changing, and how nothing stays the same forever. Water is also used to explain how our identity (who we are) is not fixed. Like water takes the shape of whatever container it is in, people can be shaped by their surroundings and experiences. In more recent times, philosophers still talk about water in many important ways. In ecological philosophy, they explore how people are connected to nature and the environment, and water is a big part of that.[338] In existentialism, water can be a symbol for things like uncertainty, deep thoughts, or the search for meaning in life. In environmental ethics, water is often discussed in debates about natural rights, fairness, and how to protect the planet. Some modern philosophers believe that water should be seen not just as a resource we use, but as a living system that should be treated with respect and care. Others study how water affects people’s lives, culture, and identity. In a type of philosophy called phenomenology, which looks at how we experience the world, water might be studied through the senses. How it feels, looks, sounds, and moves.[339]
In folklore
[change | change source]
Water has been connected to mythical creatures and spirits in many cultures around the world. Stories often tell of beings like mermaids, nymphs, kelpies, nāgas, and other water spirits that live in rivers, lakes, or the ocean. These magical beings are sometimes seen as protectors of water. They might guard a water source and punish people who harm it. In other stories, they may try to trick or lure humans into the water. Some water spirits are wise and kind, offering help or magical advice. Others are tricksters or bring bad luck.[340] In Japanese folklore, there is a creature called the kappa. Kappa are often seen as water spirits or kami in Japanese folklore. They can act in both mischievous and dangerous ways. Sometimes, they play pranks, like trying to peek under people's clothing near the water. But in other stories, they can be harmful, even trying to drown people or animals or scare children. Even though they can be scary, not all kappa are mean. Some are shown to be friendly and can even help humans.[341] In Celtic legends, water fairies are magical beings that live in rivers, lakes, springs, and wells. They are often connected to nature. They are believed to guard the water and its power. These fairies can be kind and helpful, but they can also be tricky or dangerous if they are disrespected.[342] In parts of Africa, people believe in Mami Wata, a powerful water spirit who can appear as a beautiful woman or a mermaid. She is seen as a guardian of rivers and oceans. Mami Wata protects the natural world. She can bring blessings to those who honor her. But if someone disrespects her, pollutes the water, or breaks a promise, she may punish them. [343]In Greek mythology, sirens were dangerous creatures who lived on rocky islands in the sea. They looked like part woman and part bird, but later stories showed them to be more like mermaids. Sirens had beautiful voices and sang songs so sweet that sailors could not resist. The music would lure the sailors closer and closer until their ships crashed on the rocks.[344]
In many myths and folktales, water is seen as a gateway or border between different worlds. Rivers, lakes, and oceans are often shown as dividing lines between the world of the living and the dead, or between the human world and the spirit world. In Greek mythology, there is a river called the Styx that separates the world of the living from the underworld. When someone dies, their soul must cross the river to reach the land of the dead. A boatman named Charon ferries the souls across, but only if they have a coin to pay him.[345] In Celtic stories, lakes and misty waters are often seen as gateways to the Otherworld, a magical realm where spirits and fairies live. Some tales say that if you walk into the mist on a quiet lake, you might disappear into another world. The boundary between the worlds is thin near water, especially at dawn or dusk.[346][347] In Japanese folklore, there is a belief that spirits of the dead must cross a river called the Sanzu River to reach the afterlife. Like the Greek Styx, it is a border between the living and the dead. Depending on how someone lived their life, they might cross on a bridge, wade through shallow water, or struggle through deep, rough currents.[348] In some Native American traditions, water is a pathway between the physical world and the spirit world. Shamans or spiritual leaders may enter a trance near a river or waterfall to connect with spirits.[349]
In many cultures, water plays an important role in myths about creation and destruction. In Babylonian mythology, the world began from a vast, dark ocean. Two great water beings, Apsu (fresh water) and Tiamat (salt water), came together to create the first gods. Later, Tiamat became angry and turned into a monster to destroy them. One of the gods, Marduk, defeated her and used her body to form the sky and the earth.[350] In the Bible, there is the story of Noah's Ark, where God sends a great flood to destroy the world because of human wickedness. The floodwaters cover the earth, wiping out nearly everything. But after the water goes down, life begins again.[351] In Hindu mythology, the god Vishnu rests on a cosmic ocean before the world is created. From his navel grows a lotus flower, and from that flower comes Brahma, the creator god. Later, when the world becomes evil, Vishnu sends great floods to destroy it and prepare for a new age.[352] In Norse mythology, at the end of the world, called Ragnarök, giant waves and floods cover the earth as the sea serpent Jörmungandr rises from the ocean. Many gods die, and the world is destroyed in water and fire. But after the flood, the world slowly rises again, green and peaceful, ready for a new beginning.[353]
In many myths and legends, water is linked to healing and change. People have long believed that special places like sacred springs, wells, and fountains have magical or healing powers. In these stories, someone who drinks the water or bathes in it might be cured of an illness, feel stronger, or even gain special abilities. In Celtic folklore, there are many stories about sacred wells and springs. These places were believed to be blessed by spirits or ancient gods. People would visit them to wash their wounds or drink the water, hoping to be healed. Some wells were also believed to grant visions or wisdom if the visitor was pure of heart.[354][355] In Christian tradition, the waters of Lourdes in France are said to have healing powers. A young girl named Bernadette saw visions of the Virgin Mary near a spring. Since then, millions of people have traveled to Lourdes to bathe in the water or collect it, praying for cures to sickness or pain.[356] In Japanese mythology, there are stories of people bathing in hot springs or sacred waterfalls to purify their bodies and spirits. These waters are connected to nature spirits called kami, and being near them can bring peace, health, and even a new beginning.[357] In Greek myths, the fountain of youth or magical springs could restore a person’s youth and beauty. Heroes sometimes went on long journeys to find such water.[358] One famous example is the River Lethe, where drinking the water caused people to forget their past, offering a kind of spiritual reset or rebirth.[359]
In art
[change | change source]

Water has been a powerful symbol in art for thousands of years. Artists use water to show many different feelings and ideas, such as peace, change, danger, or mystery. In paintings, water often appears as oceans, rivers, rain, or even tears. It can be calm and still, like a quiet lake, or wild and strong, like crashing waves. These images help people feel something when they look at the artwork. For example, a peaceful painting of a pond might make someone feel relaxed, while a stormy sea might show fear or struggle.[360][361]
In many cultures, water is also used in art to show life and rebirth. In religious art, water is sometimes shown as holy, like in Christian paintings of baptisms, where water is used to wash away sins and begin a new life.[362] In Hindu and Buddhist art, rivers like the Ganges are drawn or carved into temples to show purity and connection to the divine.[363][364] In ancient times, water also appeared in art to show myths and legends. In Greek and Roman art, gods like Poseidon or Neptune were often shown holding tridents and riding waves or sea creatures.[365] In Egyptian art, the Nile River was a symbol of life and was often painted in scenes of farming, fishing, and ceremonies.[366]
One famous example of water in art is “The Great Wave off Kanagawa” by the Japanese artist Hokusai. This woodblock print shows a huge, powerful wave towering over small boats, with Mount Fuji in the background. The wave looks alive, with curling foam like claws, ready to crash down. Even though the image is full of movement and danger, it is also beautifully balanced and carefully designed. Hokusai may have used the sea not just to show nature’s power, but also the strength and struggle of people facing it. This artwork has become one of the most well-known in the world.[367] Another famous painting that shows water is “Water Lilies” by the French artist Claude Monet. Monet painted many versions of this scene, showing a quiet pond with lily pads, flowers, and reflections of trees and sky on the surface of the water. The soft colors and blurred brushstrokes make the paintings feel peaceful and dreamlike. His paintings help people feel calm and connected to nature, like they are standing next to the pond themselves.[368][369]
Artists also use water in more practical ways. Watercolor painting, for example, uses water to blend colors in soft, flowing shapes. This kind of painting is known for its light, dreamy look. It is perfect for painting things like clouds, rain, or gentle waves.[370] Some modern artists even create art with water itself, such as fountains, pools, or water installations that change with movement and time. These works let people see, hear, and even touch the water, making the experience feel alive and special.[371][372]
In literature
[change | change source]
Water shows up in many stories, poems, and myths from all over the world. In books and other writing, water often stands for feelings, life, change, or the unknown.[373][374] One well-known example is in the book The Old Man and the Sea by Ernest Hemingway. In this story, an old fisherman goes out into the ocean by himself and tries to catch a huge fish. The sea is more than just the place where the story happens. As the fisherman fights to catch the fish, the story talks about human strength, being alone, and having respect for nature. The water shows both danger and deep wisdom.[375]
In Moby-Dick by Herman Melville, the ocean is a big part of the story. The book is about Captain Ahab and his crew as they sail across the sea to find a giant white whale named Moby Dick. The ocean is not just where the story takes place, it also stands for big ideas like the unknown, the power of nature, and life’s deepest questions. Captain Ahab becomes obsessed with hunting the whale, and this makes the sea feel dangerous and full of madness.[376] In The Odyssey by Homer, water is a big part of the story. Odysseus, the main hero, spends many years sailing across the sea to get home after the Trojan War. Along the way, he faces storms, sea monsters, and powerful gods. Each time he travels by water, he faces new problems that test how brave and smart he is. The sea is more than just something to cross, it is a place of adventure, danger, and change. It helps Odysseus grow into a wiser and stronger person. This ancient story shows that water, especially the ocean, was seen as a powerful and magical force, full of meaning and mystery.[377]
In The Secret Garden by Frances Hodgson Burnett, water is shown in a soft and peaceful way. There are moments when rain falls and helps bring the garden back to life. The rain makes the flowers grow and the air feel fresh and clean. For the characters in the story, hearing the rain and feeling the wet earth become signs that things are getting better. The water helps turn the garden from a quiet, lonely place into one that is full of beauty and happiness.[378] In Huckleberry Finn by Mark Twain, the Mississippi River is very important to the story. Huck and Jim, who is an escaped slave, travel down the river on a raft. The river stands for freedom and escape. As they float along, they have many adventures, meet different people, and talk about big ideas like right and wrong, and what it means to be a friend. The river is always moving and changing, just like their journey. It gives them time and space to think and grow. Twain uses the river not just as a way to get from place to place, but as a symbol of the characters’ path to better understand themselves and the world around them.[379]
In poetry, water is often used to show feelings and ideas. In the poem The Rime of the Ancient Mariner by Samuel Taylor Coleridge, a sailor tells a strange and sad story about being lost at sea. The huge ocean around him becomes a symbol of guilt, punishment, and mystery. One famous line from the poem is, “Water, water, every where, And all the boards did shrink; Water, water, every where, nor any drop to drink.” This shows how the ocean, which usually gives life, can also be dangerous and cruel. The poem uses water to teach a lesson, that people must respect nature and the spiritual world.[380] In many myths and legends, water is part of a journey to another world. In the Greek story of Orpheus and Eurydice, Orpheus has to cross a river to enter the underworld and try to bring back his lost love. The river is a border between life and death, showing that water can mark the edge of what people understand.[381] In an African story about Mami Wata, a water spirit appears in dreams and visions. She might bring healing, beauty, or riches, but she must be treated with respect.[343]
In movies
[change | change source]
Water is often used in movies to create powerful emotions, build tension, or act as an challenge for the characters. Directors use water in different ways. It can show danger, mystery, or fear, but it can also show peace, life, or change. In adventure and action movies, water can be exciting or dangerous. It can appear in scenes with storms, floods, or rescues from rivers or oceans.[382] One well-known example is Titanic (1997), where the ocean is not only the setting but also a major cause of the disaster. At first, the sea looks calm and beautiful, but later, the cold water becomes a threat as the ship sinks.[383] In fantasy and science fiction movies, water can be strange or magical. For example, in Avatar: The Way of Water, the ocean is a place full of unusual creatures and emotional moments. It is shown as both a home and a challenge for the characters.[384]
In the animated movie Moana, water is shown as something that can move on its own and help the main character. The ocean guides Moana as she tries to save her island. It helps her when she is scared and keeps her safe from harm. In the movie, water acts like a helper. It also links Moana to her ancestors, who also sailed the oceans. The ocean in this story is not something to be afraid of but something to understand and rely on. In Finding Nemo (2003), the entire story takes place underwater. The ocean is full of sea life, reefs, and dangers. It shows that water can be both fun and dangerous. The underwater setting is important to the whole story. In Cast Away (2000), the ocean separates the main character from other people after a plane crash. The ocean becomes a challenge he must face, and it is also the way he must return to his normal life.
Water is also used in movies to show feelings or deeper meanings. In many dramas, rain can show sadness, being alone, or a big change in the story. For example, in The Notebook, the rain scene is full of strong emotion and shows an important moment between two characters. Water can also stand for a new beginning or change. In horror movies, water can make scenes more tense or scary. Dark lakes, bathtubs, or storms can trap people or hide something dangerous. In Jaws, for example, the ocean looks calm, but a shark is hiding below. People swim without knowing the danger, which creates fear and suspense.[385] Water also works well in movies because it moves, reflects light, and makes sounds. Whether it is big waves, gentle rain, or quiet ponds, water adds something special to a scene. It helps tell the story in a visual way, sometimes more clearly than words can. This makes water one of the most useful natural elements in filmmaking.
Dihydrogen monoxide parody
[change | change source]The dihydrogen monoxide parody began as a funny joke in the early 1990s. It was meant to show how using big science words can confuse people and make safe things sound dangerous. "Dihydrogen monoxide" is just a fancy way of saying water. The word “dihydrogen” means two hydrogen atoms, and “monoxide” means one oxygen atom. That adds up to H₂O, which is the chemical formula for water. Even though it is just water, calling it “dihydrogen monoxide” makes it sound like a harmful chemical. This joke was used to show that many people do not always understand science words, and they can be tricked if something is explained in a dramatic or serious-sounding way.[386]
One of the earliest and most famous uses of this joke happened in 1997, when a high school student named Nathan Zohner gave a science presentation titled "How Gullible Are We?". In his report, he described all the dangers of “dihydrogen monoxide”. Like causing burns (as steam), contributing to erosion, being found in cancer cells, and even causing death when inhaled. He did not say that it was just water until the very end. Out of 50 students who listened to his presentation, 43 voted to ban the chemical. Nathan’s project became famous, and it showed how people can be easily tricked if they do not have enough scientific knowledge or critical thinking.[386]
Before that, versions of the joke had appeared in internet forums and emails, especially as the internet was growing in the 1990s. People used the parody to make fun of how the media, politicians, or even environmental activists might sometimes overreact to scientific information without fully understanding it. Over time, the joke spread widely and became part of internet culture. It is often used today in classrooms, debates, or websites to teach people to read carefully and to question information that sounds too dramatic or scary. The DHMO parody continues to be shared today, often as a reminder that how we present information really matters. If something is said with the right tone, long words, or half-truths, people might believe anything, even that water is dangerous. It is a funny but important lesson in science literacy, skepticism, and not jumping to conclusions without understanding the full picture. The website DHMO.org is a joke website which lists the harmful effects of water (DHMO), answers questions, and calls for it to be banned, among other things.[386][387]
In music
[change | change source]Water has always been a big inspiration in music because it connects to so many feelings and ideas. People all over the world use water as a symbol in songs to show emotions like peace, sadness, change, power, or even mystery. Just like water can be calm or stormy, music about water can be soft and gentle or loud and intense. For example, a calm river or gentle rain might be used to create a peaceful feeling, while crashing waves or a flood might show strong emotions like anger, heartbreak, or chaos. Water can represent the flow of life, the passage of time, or the feeling of being overwhelmed by something big.[388]
In classical music, composers have often used water to paint pictures with sound. For example, famous composers like Claude Debussy wrote pieces such as La Mer (The Sea), where the music was inspired by the sea.[389] Another example is Frédéric Chopin’s "Raindrop Prelude", where repeating notes sound like gentle raindrops falling.[390] These pieces do not use words, but the music still makes listeners feel like they are near water. In modern times, pop, rock, and folk songs also use water in their lyrics. Songs like Bridge Over Troubled Water by Simon & Garfunkel or River by Joni Mitchell use rivers and storms as ways to talk about emotions and life problems. In these songs, water can be both the problem and the solution. It can pull someone down, or it can wash their pain away.[391][392]
In many cultures, water is tied to spiritual or emotional cleansing, so music about water is often healing or thoughtful. In African, Asian, and Indigenous traditions, water songs are sometimes sung during rituals, rain dances, or prayers, asking nature for help or giving thanks for life.[393][394] In blues music, rivers often appear as symbols of sorrow or escape, especially in songs from the American South, where the Mississippi River became a symbol of travel, freedom, and sometimes grief.[395] In reggae and Caribbean music, the sea and rain show up in joyful, laid-back songs that celebrate nature and the rhythm of island life.[396][397]
Water even affects how music sounds. In some electronic or ambient music, water sounds like waves, rain, or dripping are added to create a relaxing background. These sounds are used in meditation music or sleep playlists because they help people feel calm and safe.[398] Music therapists also use water sounds to help people reduce stress or connect with their emotions.[399]
Related pages
[change | change source]Notes
[change | change source]- ↑ 1.0 1.1 Vienna Standard Mean Ocean Water (VSMOW), used for calibration, melts at 273.1500089(10) K (0.000089(10) °C, and boils at 373.1339 K (99.9839 °C). Other isotopic compositions melt or boil at slightly different temperatures.
- ↑ A commonly quoted value of 15.7 used mainly in organic chemistry for the pKa of water is incorrect.[10][11]
References
[change | change source]- ↑ "naming molecular compounds". www.iun.edu. Archived from the original on 24 September 2018. Retrieved 1 October 2018.
Sometimes these compounds have generic or common names (e.g., H2O is "water") and they also have systematic names (e.g., H2O, dihydrogen monoxide).
- ↑ "Definition of Hydrol". Merriam-Webster.
- ↑ Riddick 1970, Table of Physical Properties, Water 0b. pg 67-8.
- ↑ Lide 2003, Properties of Ice and Supercooled Water in Section 6.
- ↑ Water in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov (retrieved 2016-5-27)
- ↑ 6.0 6.1 Anatolievich, Kiper Ruslan. "Properties of substance: water". Archived from the original on 2014-06-02. Retrieved 2021-02-07.
- ↑ Lide 2003, Vapor Pressure of Water From 0 to 370° C in Sec. 6.
- ↑ Lide 2003, Chapter 8: Dissociation Constants of Inorganic Acids and Bases.
- ↑ Weingärtner et al. 2016, p. 13.
- ↑ "What is the pKa of Water". University of California, Davis. 2015-08-09. Archived from the original on 2016-02-14. Retrieved 2020-09-12.
- ↑ Silverstein, Todd P.; Heller, Stephen T. (17 April 2017). "pKa Values in the Undergraduate Curriculum: What Is the Real pKa of Water?". Journal of Chemical Education. 94 (6): 690–695. Bibcode:2017JChEd..94..690S. doi:10.1021/acs.jchemed.6b00623. ISSN 0021-9584.
- ↑ Ramires, Maria L. V.; Castro, Carlos A. Nieto de; Nagasaka, Yuchi; Nagashima, Akira; Assael, Marc J.; Wakeham, William A. (1995-05-01). "Standard Reference Data for the Thermal Conductivity of Water". Journal of Physical and Chemical Reference Data. 24 (3): 1377–1381. Bibcode:1995JPCRD..24.1377R. doi:10.1063/1.555963. ISSN 0047-2689.
- ↑ Lide 2003, 8—Concentrative Properties of Aqueous Solutions: Density, Refractive Index, Freezing Point Depression, and Viscosity.
- ↑ Lide 2003, 6.186.
- ↑ Lide 2003, 9—Dipole Moments.
- ↑ 16.0 16.1 16.2 Water in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD), http://webbook.nist.gov (retrieved 2014-06-01)
- ↑ "United Nations". Un.org. 2005-03-22. Retrieved 2010-07-25.
- ↑ "Etymology of "water" by etymonline". etymonline. Retrieved 2025-05-03.
- ↑ 19.0 19.1 "How Much Water is There on Earth?". Water Science School. United States Geological Survey, U.S. Department of the Interior. 13 November 2019. Archived from the original on 9 June 2022. Retrieved 8 June 2022.
- ↑ 20.0 20.1 "Distribution of Water on the Earth's Surface | EARTH 103: Earth in the Future". www.e-education.psu.edu. Retrieved 2025-05-03.
- ↑ Gleick, P.H., ed. (1993). Water in Crisis: A Guide to the World's Freshwater Resources. Oxford University Press. p. 13, Table 2.1 "Water reserves on the earth". Archived from the original on 8 April 2013.
- ↑ Water Vapor in the Climate System Archived 20 March 2007 at the Wayback Machine, Special Report, [AGU], December 1995 (linked 4/2007). Vital Water Archived 20 February 2008 at the Wayback Machine UNEP.
- ↑ "The anomalous properties of water". ergodic.ugr.es. Retrieved 2025-05-03.
- ↑ 24.0 24.1 "8(a) Physical Properties of Water". physicalgeography.net. 2011. Retrieved August 31, 2011.
pan
- ↑ "Why Does Ice Float? | The Children's Museum of Indianapolis". www.childrensmuseum.org. Retrieved 2025-05-03.
- ↑ "2.14: Water - High Heat Capacity". Biology LibreTexts. 2018-07-05. Retrieved 2025-05-03.
- ↑ 27.0 27.1 von Schuckmann, K.; Cheng, L.; Palmer, M. D.; Hansen, J.; et al. (7 September 2020). "Heat stored in the Earth system: where does the energy go?". Earth System Science Data. 12 (3): 2013–2041. Bibcode:2020ESSD...12.2013V. doi:10.5194/essd-12-2013-2020. hdl:20.500.11850/443809. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ "Surface tension is a contractive tendency of the surface of a liquid that allows it to resist an external force". Boundless. Archived from the original on June 3, 2016. Retrieved December 25, 2016.
- ↑ 29.0 29.1 29.2 29.3 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 620. ISBN 978-0-08-037941-8.
- ↑ 30.0 30.1 30.2 30.3 "Water, the Universal Solvent". USGS. Archived from the original on 9 July 2017. Retrieved 27 June 2017.
- ↑ 31.0 31.1 31.2 31.3 "Solvent properties of water". Khan Academy.
- ↑ 32.0 32.1 "Water Q&A: Why is water the "universal solvent"?". Water Science School. United States Geological Survey, U.S. Department of the Interior. 20 June 2019. Archived from the original on 6 February 2021. Retrieved 15 January 2021.
- ↑ 33.0 33.1 33.2 "11.9: Water - An Extraordinary Substance". Chemistry LibreTexts. 2017-06-29. Retrieved 2024-06-22.
- ↑ "What makes water an important molecule for life?". University of Nevada, Reno. Retrieved 2025-05-05.
- ↑ U.S. Geological Survey (2 June 2023). "Water in the Human Body". Water Science School. Retrieved 30 April 2025.
- ↑ Hall, D.O. (2001). Photosynthesis, Sixth edition. University of Cambridge. ISBN 0-521-64497-6. Archived from the original on 5 October 2023. Retrieved 26 August 2023.
- ↑ 37.0 37.1 37.2 37.3 37.4 37.5 37.6 37.7 37.8 Reece, Jane B. (2013). Campbell Biology (10th ed.). Pearson. p. 48. ISBN 978-0-321-77565-8.
- ↑ "Economic valuation of water resources in agriculture". www.fao.org. Retrieved 2025-05-05.
- ↑ Ritchie, Hannah; Roser, Max (2018-07-01). "Water Use and Stress". Our World in Data.
- ↑ Troell, Max; Naylor, Rosamond L.; Metian, Marc; Beveridge, Malcolm; Tyedmers, Peter H.; Folke, Carl; Arrow, Kenneth J.; Barrett, Scott; Crépin, Anne-Sophie; Ehrlich, Paul R.; Gren, Åsa (16 September 2014). "Does aquaculture add resilience to the global food system?". Proceedings of the National Academy of Sciences. 111 (37): 13257–13263. Bibcode:2014PNAS..11113257T. doi:10.1073/pnas.1404067111. ISSN 0027-8424. PMC 4169979. PMID 25136111.
- ↑ "Global Freight Demand to Triple by 2050". The Maritime Executive. May 27, 2019.
- ↑ "Hydropower Basics". Energy.gov. Retrieved 2025-05-03.
- ↑ "Water sports". Dictionary.com. Retrieved 2025-05-03.
- ↑ Pollock, Susan (1999), Ancient Mesopotamia. The Eden that never was, Case Studies in Early Societies, Cambridge: Cambridge University Press, p. 1, ISBN 978-0-521-57568-3
- ↑ Shaw, Ian, ed. (2003). The Oxford History of Ancient Egypt. Oxford: Oxford University Press. ISBN 978-0-19-280458-7.
- ↑ Williams, Brian (2016). Daily Life in the Indus Valley Civilization. Raintree. p. 6. ISBN 978-1406298574.
- ↑ "Tiber River". Encyclopædia Britannica. 2006.
- ↑ "The Hudson River Estuary". Life.bio.sunysb.edu. Stony Brook University. Archived from the original on June 4, 2017. Retrieved August 20, 2011.
- ↑ Asariotis, Regina; Benamara, Hassiba; Mohos-Naray, Viktoria (December 2017). Port Industry Survey on Climate Change Impacts and Adaptation (PDF) (Report). UN Conference on Trade and Development. Archived (PDF) from the original on 2020-11-25.
- ↑ "Maritime ports freight and passenger statistics" (PDF). Eurostat. Archived (PDF) from the original on 2017-07-22. Retrieved 18 June 2020.
- ↑ "Singapore named top maritime capital of the world for 3rd consecutive time". The Straits Times. 26 April 2017.
- ↑ Rijsberman, Frank R. (2006). "Water scarcity: Fact or fiction?". Agricultural Water Management. 80 (1–3): 5–22. Bibcode:2006AgWM...80....5R. doi:10.1016/j.agwat.2005.07.001.
- ↑ Bibring, Jean-Pierre; Langevin, Yves; Poulet, François; Gendrin, Aline; Gondet, Brigitte; Berthé, Michel; Soufflot, Alain; Drossart, Pierre; Combes, Michel (April 2004). "Perennial water ice identified in the south polar cap of Mars". Nature. 428 (6983): 627–630. Bibcode:2004Natur.428..627B. doi:10.1038/nature02461. ISSN 0028-0836. PMID 15024393. S2CID 4373206.
- ↑ Anderson, Gina (2015-09-28). "NASA Confirms Evidence That Liquid Water Flows on Today's Mars". NASA. Retrieved 2020-09-12.
- ↑ "NASA Space Assets Detect Ocean inside Saturn Moon". NASA/JPL. Retrieved 2020-09-12.
- ↑ Iess, L.; Stevenson, D. J.; Parisi, M.; Hemingway, D.; Jacobson, R. A.; Lunine, J. I.; Nimmo, F.; Armstrong, J. W.; Asmar, S. W. (2014-04-04). "The Gravity Field and Interior Structure of Enceladus". Science. 344 (6179): 78–80. Bibcode:2014Sci...344...78I. doi:10.1126/science.1250551. ISSN 0036-8075. PMID 24700854. S2CID 28990283.
- ↑ Dunaeva, A. N.; Kronrod, V. A.; Kuskov, O. L. (2016). "Physico-chemical models of the internal structure of partially differentiated Titan". Geochemistry International. 54 (1): 27–47. doi:10.1134/s0016702916010043. ISSN 0016-7029. S2CID 130371184.
- ↑ Hanslmeier, Arnold. (2011). Water in the universe. Dordrecht: Springer. ISBN 978-90-481-9984-6. OCLC 670074794.
- ↑ Garner, Rob (2015-05-06). "Hubble Traces Subtle Signals of Water on Hazy Worlds". NASA. Retrieved 2020-09-11.
- ↑ Melnick, Gary, Harvard-Smithsonian Center for Astrophysics and Neufeld, David, Johns Hopkins University quoted in: "Discover of water vapor near Orion nebula suggests possible origin of H20 in Solar System". The Harvard University Gazette. 1998. Archived from the original on 16 January 2000. "Space cloud holds enough water to fill Earth's oceans 1 million times". Headlines@Hopkins, JHU. 9 April 1998. Archived from the original on 9 November 2007. Retrieved 21 April 2007.
- ↑ "1.24: Big Bang Nucleosynthesis - Observations". Physics LibreTexts. 2021-10-25. Retrieved 2025-05-22.
- ↑ "Early Universe - NASA Science". 2022-06-01. Retrieved 2025-05-22.
- ↑ "Stars - NASA Science". 2023-06-09. Retrieved 2025-05-22.
- ↑ 64.0 64.1 "The Origin of Water - and Other Pre-biotic Molecules - in Planetary Systems". spherex.caltech.edu. Retrieved 2025-05-06.
- ↑ Dulieu, F.; Amiaud, L.; Congiu, E.; Fillion, J.-H.; Matar, E.; Momeni, A.; Pirronello, V.; Lemaire, J. L. (2010-03-01). "Experimental evidence for water formation on interstellar dust grains by hydrogen and oxygen atoms". Astronomy & Astrophysics. 512: A30. doi:10.1051/0004-6361/200912079. ISSN 0004-6361.
- ↑ "Astronomers reveal a new link between water and planet formation | ALMA Observatory". Retrieved 2025-05-22.
- ↑ [email protected]. "Astronomers reveal a new link between water and planet formation". www.eso.org. Retrieved 2025-05-22.
- ↑ 68.0 68.1 68.2 68.3 68.4 68.5 68.6 "Tour of Water in the Solar System | U.S. Geological Survey". www.usgs.gov. Retrieved 2025-05-06.
- ↑ "Astronomers Find Largest, Most Distant Reservoir of Water". NASA Jet Propulsion Laboratory (JPL). Retrieved 2025-05-22.
- ↑ US Department of Commerce, National Oceanic and Atmospheric Administration. "Are there oceans on other planets?". oceanservice.noaa.gov. Retrieved 2025-05-04.
- ↑ Lammer, H.; Bredehöft, J. H.; Coustenis, A.; Khodachenko, M. L.; Kaltenegger, L.; Grasset, O.; Prieur, D.; Raulin, F.; Ehrenfreund, P.; Yamauchi, M.; Wahlund, J.-E. (2009). "What makes a planet habitable?". The Astronomy and Astrophysics Review. 17 (2): 181–249. doi:10.1007/s00159-009-0019-z. ISSN 0935-4956.
- ↑ "Origin of Water on Earth" (PDF). Harvard University. Retrieved 30 April 2025.
- ↑ Genda, Hidenori (2016). "Origin of Earth's oceans: An assessment of the total amount, history and supply of water". Geochemical Journal. 50 (1): 27–42. doi:10.2343/geochemj.2.0398.
- ↑ "Where did Earths water come from". Astronomy.com. Retrieved 2020-07-16.
- ↑ Henbest, Nigel. "Birth of the planets". New Scientist. Retrieved 2025-05-04.
- ↑ Dublin, Trinity College. "Comets or volcanoes? Where did Earth's water come from?". www.tcd.ie. Retrieved 2025-05-04.
- ↑ Balmer, William (2023-04-27). "Did early Earth order delivery, or did it make its oceans at home?". astrobites.org. Retrieved 2025-05-04.
- ↑ published, Charles Q. Choi (2014-12-10). "Most of Earth's Water Came from Asteroids, Not Comets". Space. Retrieved 2025-05-04.
- ↑ Gomes, R.; Levison, H. F.; Tsiganis, K.; Morbidelli, A. (2005). "Origin of the cataclysmic Late Heavy Bombardment period of the terrestrial planets". Nature. 435 (7041): 466–469. doi:10.1038/nature03676. ISSN 1476-4687.
- ↑ Daly, R. Terik; Schultz, Peter H. (2018). "The delivery of water by impacts from planetary accretion to present". Science Advances. 4 (4): eaar2632. doi:10.1126/sciadv.aar2632. ISSN 2375-2548. PMC 5916508. PMID 29707636.
- ↑ Pepin, Robert O. 1991. On the origin and early evolution of terrestrial planet atmospheres and meteoritic volatiles. Icarus 92 (1): 2–79. [1]
- ↑ Pinti, Daniele L.; Arndt, Nicholas (2014), "Oceans, Origin of", Encyclopedia of Astrobiology, Springer Berlin Heidelberg, pp. 1–5, doi:10.1007/978-3-642-27833-4_1098-4, ISBN 978-3-642-27833-4
- ↑ O'Neil, Jonathan; Carlson, Richard W.; Paquette, Jean-Louis; Francis, Don (November 2012). "Formation age and metamorphic history of the Nuvvuagittuq Greenstone Belt" (PDF). Precambrian Research. 220–221: 23–44. Bibcode:2012PreR..220...23O. doi:10.1016/j.precamres.2012.07.009. ISSN 0301-9268.
- ↑ Piani, Laurette (28 August 2020). "Earth's water may have been inherited from material similar to enstatite chondrite meteorites". Science. 369 (6507): 1110–1113. Bibcode:2020Sci...369.1110P. doi:10.1126/science.aba1948. PMID 32855337. S2CID 221342529. Retrieved 28 August 2020.
- ↑ Washington University in St. Louis (27 August 2020). "Meteorite study suggests Earth may have been wet since it formed - Enstatite chondrite meteorites, once considered 'dry,' contain enough water to fill the oceans -- and then some". EurekAlert!. Archived from the original on 30 October 2020. Retrieved 28 August 2020.
- ↑ American Association for the Advancement of Science (27 August 2020). "Unexpected abundance of hydrogen in meteorites reveals the origin of Earth's water". EurekAlert!. Archived from the original on 28 October 2020. Retrieved 28 August 2020.
- ↑ Wilde, S.A.; Valley, J.W.; Peck, W.H.; Graham, C.M. (2001). "Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 nGyr ago" (PDF). Nature. 409 (6817): 175–8. Bibcode:2001Natur.409..175W. doi:10.1038/35051550. PMID 11196637. S2CID 4319774.
- ↑ "ANU - Research School of Earth Sciences - ANU College of Science - Harrison". Ses.anu.edu.au. Archived from the original on 2006-06-21. Retrieved 2009-08-20.
- ↑ "ANU - OVC - MEDIA - MEDIA RELEASES - 2005 - NOVEMBER - 181105HARRISONCONTINENTS". Info.anu.edu.au. Retrieved 2009-08-20.
- ↑ "A Cool Early Earth". Geology.wisc.edu. Archived from the original on 2013-06-16. Retrieved 2009-08-20.
- ↑ Valley, John W.; Peck, William H.; King, Elizabeth M.; Wilde, Simon A. (2002). "A cool early Earth". Geology. 30 (4): 351. Bibcode:2002Geo....30..351V. doi:10.1130/0091-7613(2002)030<0351:ACEE>2.0.CO;2. ISSN 0091-7613.
- ↑ Nebel, Oliver; Rapp, Robert P.; Yaxley, Gregory M. (2014-03-01). "The role of detrital zircons in Hadean crustal research". Lithos. 190–191: 313–327. doi:10.1016/j.lithos.2013.12.010. ISSN 0024-4937.
- ↑ Chang, Kenneth (2008-12-02). "A New Picture of the Early Earth". The New York Times. Retrieved 2010-05-20.
- ↑ Roberts, Richard G. (2014). "Human evolution: Just add water". Nature. 507 (7492): 303–304. doi:10.1038/507303a. ISSN 1476-4687.
- ↑ "What was the Neolithic Revolution?". Culture. 2025-05-23. Retrieved 2025-05-23.
- ↑ Sahrhage, Dietrich (2008), Selin, Helaine (ed.), "Fishing in the Stone Age", Encyclopaedia of the History of Science, Technology, and Medicine in Non-Western Cultures, Dordrecht: Springer Netherlands, pp. 935–939, doi:10.1007/978-1-4020-4425-0_8594, ISBN 978-1-4020-4425-0, retrieved 2025-05-23
- ↑ "Ancient canoes hint at bustling trade in Mediterranean 7000 years ago". New Scientist. Retrieved 2025-05-23.
- ↑ Davenport, Ben (2013-08-09). "Ancient Mesopotamia - an overview". www.arch.cam.ac.uk. Retrieved 2025-05-23.
- ↑ "Early Agricultural Communities". education.nationalgeographic.org. Retrieved 2025-05-23.
- ↑ "Egypt and the Nile". carnegiemnh.org. Retrieved 2025-05-23.
- ↑ "Ancient Egypt facts and history". History. 2023-01-27. Retrieved 2025-05-23.
- ↑ "Who were the Indus people and how did they live?". BBC Bitesize. Retrieved 2025-05-23.
- ↑ "Indus Valley civilization". www.khanacademy.org. Retrieved 2025-05-23.
- ↑ "An Introduction to Ancient China". www.khanacademy.org. Retrieved 2025-05-23.
- ↑ "Huang He Valley". education.nationalgeographic.org. Retrieved 2025-05-23.
- ↑ Cheng, Lin sun; Brown, Kerry (2009). Berkshire encyclopedia of China: modern and historic views of the world's newest and oldest global power. Great Barrington (Mass.): Berkshire Pub. Group. ISBN 978-0-9770159-4-8.
- ↑ "Roman Aqueducts". education.nationalgeographic.org. Retrieved 2025-05-23.
- ↑ Deming, David (2020). "The Aqueducts and Water Supply of Ancient Rome". Groundwater. 58 (1): 152–161. doi:10.1111/gwat.12958. ISSN 1745-6584. PMC 7004096. PMID 31667822.
- ↑ Deming, David (2020). "The Aqueducts and Water Supply of Ancient Rome". Ground Water. 58 (1): 152–161. doi:10.1111/gwat.12958. ISSN 1745-6584. PMC 7004096. PMID 31667822.
- ↑ "Tigris-Euphrates river system - Mesopotamia, Shatt al-Arab, Basins | Britannica". www.britannica.com. Retrieved 2025-05-23.
- ↑ "Euphrates River: A Cradle of Civilizations - Ocean Info". oceaninfo.com. 2024-02-01. Retrieved 2025-05-23.
- ↑ "The History of Ancient Nubia | Institute for the Study of Ancient Cultures". isac.uchicago.edu. Retrieved 2025-05-23.
- ↑ "Nubia and Ancient Egypt". www.khanacademy.org. Retrieved 2025-05-23.
- ↑ Mark, Joshua J. "Trade in Ancient Mesopotamia". World History Encyclopedia. Retrieved 2025-05-23.
- ↑ "First Rulers of the Mediterranean". education.nationalgeographic.org. Retrieved 2025-05-23.
- ↑ Holst, Sanford (2011). Phoenician secrets: exploring the ancient Mediterranean (1st ed.). Los Angeles: Santorini Books. ISBN 978-0-9833279-0-5.
- ↑ Elayi, Josette (2018). The Phoenicians. Atlanta: Lockwood Press. ISBN 978-1-937040-82-6.
- ↑ Cartwright, Mark. "Trade in the Phoenician World". World History Encyclopedia. Retrieved 2025-05-23.
- ↑ "SPP Häfen: The Rhine as a European transportation route". www.spp-haefen.de. Retrieved 2025-05-23.
- ↑ "The Seine is at the center of the 2024 Olympics. Here's how the iconic river shaped Paris". Premium. 2025-05-23. Retrieved 2025-05-23.
- ↑ "Venice | Silk Roads Programme". en.unesco.org. Retrieved 2025-05-23.
- ↑ "Genoa". whc.unesco.org. Retrieved 2025-05-23.
- ↑ "3.1: Age of Exploration". Humanities LibreTexts. 2021-01-15. Retrieved 2025-05-23.
- ↑ "Columbian Exchange". www.khanacademy.org. Retrieved 2025-05-23.
- ↑ "The British Empire - trade and merchant shipping - Industrial Britain, 1750-1900 overview - OCR B - GCSE History Revision - OCR B". BBC Bitesize. Retrieved 2025-05-23.
- ↑ Martins, Kim. "Dutch East India Company". World History Encyclopedia. Retrieved 2025-05-23.
- ↑ Sampath, Shrikanth; Khedr, Anwar; Qamar, Shahraz; Tekin, Aysun; Singh, Romil; Green, Ronya; Kashyap, Rahul (2021). "Pandemics Throughout the History". Cureus. 13 (9): e18136. doi:10.7759/cureus.18136. ISSN 2168-8184. PMC 8525686. PMID 34692344.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ↑ Committee, National Research Council (US) Safe Drinking Water (1977), "Historical Note", Drinking Water and Health: Volume 1, National Academies Press (US), retrieved 2025-05-23
- ↑ Cipolla, Carlo M. (1980). Before the Industrial Revolution: European society and economy, 1000-1700 (in engita) (2nd ed.). New York: Norton. ISBN 978-0-393-01343-6.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Tulchinsky, Theodore H. (2018), "John Snow, Cholera, the Broad Street Pump; Waterborne Diseases Then and Now", Case Studies in Public Health, Elsevier, pp. 77–99, doi:10.1016/B978-0-12-804571-8.00017-2, ISBN 978-0-12-804571-8, PMC 7150208, retrieved 2025-05-23
- ↑ 131.0 131.1 "Thales of Miletus | Internet Encyclopedia of Philosophy". Retrieved 2025-05-25.
- ↑ Kuroki, Hidetaka (2016). "How did Archimedes discover the law of buoyancy by experiment?". Frontiers of Mechanical Engineering. 11 (1): 26–32. doi:10.1007/s11465-016-0368-z.
- ↑ Ivey, Greg; Jones, Nicole L.; Broomhall, Susan (2019-04-04). "How Leonardo da Vinci, 'Master of Water', explored the power and beauty of its flow". The Conversation. Retrieved 2025-05-25.
- ↑ Straulino, S.; Gambi, C. M. C.; Righini, A. (2011). "Experiments on buoyancy and surface tension following Galileo Galilei". American Journal of Physics. 79 (1): 32–36. doi:10.1119/1.3492721. ISSN 0002-9505.
- ↑ "Defeated by the tides". www.mpg.de. Retrieved 2025-05-25.
- ↑ "Water is not an element: Lavoisier". web.lemoyne.edu. Retrieved 2025-05-25.
- ↑ "Chart: Globally, 70% of Freshwater is Used for Agriculture". World Bank Blogs. Retrieved 2025-05-23.
- ↑ "Where Does Our Food Come From? - University of the Potomac". Retrieved 2025-05-23.
- ↑ The State of Agricultural Commodity Markets 2024. FAO. 2024-11-29. doi:10.4060/cd2144en. ISBN 978-92-5-139061-0.
- ↑ Tidwell, J. H.; Allan, G. L. (2001). "Fish as food: aquaculture's contribution. Ecological and economic impacts and contributions of fish farming and capture fisheries". EMBO reports. 2 (11): 958–963. doi:10.1093/embo-reports/kve236. ISSN 1469-221X. PMC 1084135. PMID 11713181.
- ↑ US Department of Commerce, National Oceanic and Atmospheric Administration. "What is aquaculture?". oceanservice.noaa.gov. Retrieved 2025-05-23.
- ↑ "Shipping data: UNCTAD releases new seaborne trade statistics". UN Trade and Development (UNCTAD). Retrieved 2025-05-23.
- ↑ "Advantages And Disadvantages Of Water Transportation - Navata 2021". 2021-06-19. Retrieved 2025-05-23.
- ↑ "Main Maritime Shipping Routes and Chokepoints | Port Economics, Management and Policy". 2019-12-27. Retrieved 2025-05-23.
- ↑ "Chapter 11.2 – Ports and Economic Development | Port Economics, Management and Policy". 2019-12-23. Retrieved 2025-05-23.
- ↑ "Why ports matter for the global economy". World Bank Blogs. Retrieved 2025-05-23.
- ↑ "Chapter 11.2 – Ports and Economic Development | Port Economics, Management and Policy". 2019-12-23. Retrieved 2025-05-23.
- ↑ "Yangtze River | Location, Map, Flood, & Facts | Britannica". www.britannica.com. 2025-04-30. Retrieved 2025-05-23.
- ↑ "Canals and inland waterways - European Rivers, Navigation, Trade | Britannica". www.britannica.com. 2025-05-06. Retrieved 2025-05-23.
- ↑ "Dams". education.nationalgeographic.org. Retrieved 2025-05-23.
- ↑ "Water System | Los Angeles Department of Water and Power". www.ladwp.com. Retrieved 2025-05-23.
- ↑ "Desalination | U.S. Geological Survey". www.usgs.gov. 2018-08-30. Retrieved 2025-05-23.
- ↑ "17.3B: Wastewater and Sewage Treatment". Biology LibreTexts. 2018-07-03. Retrieved 2025-05-23.
- ↑ Omer, Sevil (2025-03-06). "Global water crisis: Facts, FAQs, and how to help". World Vision. Retrieved 2025-05-23.
- ↑ "Groundwater Decline and Depletion | U.S. Geological Survey". www.usgs.gov. 2018-10-09. Retrieved 2025-05-23.
- ↑ Nations, United. "Water – at the center of the climate crisis". United Nations. Retrieved 2025-05-23.
- ↑ "Water Pollution Definition - Types, Causes, Effects". www.nrdc.org. 2023-01-11. Retrieved 2025-05-23.
- ↑ "How rising groundwater caused by climate change could devastate coastal communities". MIT Technology Review. Retrieved 2025-05-23.
- ↑ "Flooding Facts, Causes, and Prevention | NRDC". www.nrdc.org. 2023-11-03. Retrieved 2025-05-23.
- ↑ 160.0 160.1 "Hydrogen bonding in water". www.khanacademy.org. Retrieved 2025-05-23.
- ↑ "Water Molecule Structure: The Bent Shape of Water". sparks.learning.asu.edu. Retrieved 2025-05-23.
- ↑ Weinhold, Frank (2005). Valency and bonding: a natural bond orbital donor-acceptor perspective. Clark R. Landis. Cambridge, UK: Cambridge University Press. ISBN 978-0-521-83128-4.
- ↑ "7.3: Hydrogen-Bonding and Water". Chemistry LibreTexts. 2013-10-03. Retrieved 2025-05-24.
- ↑ "Liquid water". water.lsbu.ac.uk. Retrieved 2025-05-24.
- ↑ PubChem. "Hydrogen Sulfide". pubchem.ncbi.nlm.nih.gov. Retrieved 2025-05-24.
- ↑ PubChem. "Hydrogen selenide". pubchem.ncbi.nlm.nih.gov. Retrieved 2025-05-24.
- ↑ PubChem. "Hydrogen telluride". pubchem.ncbi.nlm.nih.gov. Retrieved 2025-05-24.
- ↑ "Surface Tension (Water Properties) – USGS Water Science School". US Geological Survey. July 2015. Archived from the original on October 7, 2015. Retrieved November 6, 2015.
- ↑ "The strong polar bond between water molecules creates water cohesion. | U.S. Geological Survey". www.usgs.gov. 2019-06-18. Retrieved 2025-05-24.
- ↑ White, Harvey E. (1948). Modern College Physics. Van Nostrand. ISBN 978-0-442-29401-4.
- ↑ "Adhesion and Cohesion of Water | U.S. Geological Survey". www.usgs.gov. 2019-10-22. Retrieved 2025-05-24.
- ↑ "2.16: Water - Cohesive and Adhesive Properties". Biology LibreTexts. 2018-07-05. Retrieved 2025-05-24.
- ↑ "Capillary Action and Water | U.S. Geological Survey". www.usgs.gov. Retrieved 2024-04-29.
- ↑ Tree physics Archived 2013-11-28 at the Wayback Machine at "Neat, Plausible And" scientific discussion website.
- ↑ "Cohesion and adhesion of water". www.khanacademy.org. Retrieved 2025-05-24.
- ↑ Halliday, David; Resnick, Robert; Walker, Jearl (2001). Fundamentals of Physics (6th ed.). New York, NY US: John Wiley & Sons.
- ↑ "2.14: Water - High Heat Capacity". Biology LibreTexts. 2018-07-05. Retrieved 2025-05-25.
- ↑ "2.13: Water - Heat of Vaporization". Biology LibreTexts. 2018-07-05. Retrieved 2025-05-25.
- ↑ Bigg, Grant R. (2003). The Oceans and Climate, Second Edition (2 ed.). Cambridge: Cambridge University Press. doi:10.1017/cbo9781139165013. ISBN 978-1-139-16501-3.
- ↑ Jessen, C. (2000). Temperature Regulation in Humans and Other Mammals. Berlin: Springer. ISBN 978-3-540-41234-2.
- ↑ Wright, P. G.; Luck, C. P. (1984). "Do elephants need to sweat?". Journal of Zoology. 19 (4): 270–274. doi:10.1080/02541858.1984.11447892.
- ↑ "17.10: Heats of Fusion and Solidification". Chemistry LibreTexts. 2016-06-27. Retrieved 2025-05-25.
- ↑ "Yakhchal: Ancient Refrigerators". Earth Architecture. 9 September 2009.
- ↑ "Ice – a special substance". European Space Agency. 16 April 2013. Retrieved 26 April 2024.
- ↑ Harvey, Allan H. (2017). "Properties of Ice and Supercooled Water". In Haynes, William M.; Lide, David R.; Bruno, Thomas J. (eds.). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton, FL: CRC Press. ISBN 978-1-4987-5429-3.
- ↑ 186.0 186.1 Perlman, Howard. "Water Density". The USGS Water Science School. Archived from the original on 2016-06-25. Retrieved 2016-06-03.
- ↑ Deguchi, Shigeru; Tsujii, Kaoru (2007-06-19). "Supercritical water: a fascinating medium for soft matter". Soft Matter. 3 (7): 797–803. Bibcode:2007SMat....3..797D. doi:10.1039/b611584e. ISSN 1744-6848. PMID 32900070.
- ↑ "Phase Diagrams". ch302.cm.utexas.edu. Retrieved 2023-07-14.
- ↑ Murphy, D. M.; Koop, T. (1 April 2005). "Review of the vapour pressures of ice and supercooled water for atmospheric applications". Quarterly Journal of the Royal Meteorological Society. 131 (608): 1540. Bibcode:2005QJRMS.131.1539M. doi:10.1256/qj.04.94. S2CID 122365938. Archived from the original on 18 August 2020. Retrieved 31 August 2020.
- ↑ Wells, Sarah (21 January 2017). "The Beauty and Science of Snowflakes". Smithsonian Science Education Center. Archived from the original on 25 March 2020. Retrieved 25 March 2020.
- ↑ Whitten, Kenneth W.; Gailey, Kenneth D.; Davis, Raymond E. (1992). General chemistry (4th ed.). Saunders College Publishing. p. 475. ISBN 0-03-072373-6.
- ↑ Fellows, P. (Peter) (2017). "Freeze drying and freeze concentration". Food processing technology : principles and practice (4th ed.). Kent: Woodhead Publishing/Elsevier Science. pp. 929–940. ISBN 978-0081005231. OCLC 960758611.
- ↑ Debenedetti, P. G.; Stanley, H. E. (2003). "Supercooled and Glassy Water" (PDF). Physics Today. 56 (6): 40–46. Bibcode:2003PhT....56f..40D. doi:10.1063/1.1595053. Archived (PDF) from the original on 2018-11-01. Retrieved 2011-11-22.
- ↑ Oliveira, Mário J. de (2017). Equilibrium Thermodynamics. Springer. pp. 120–124. ISBN 978-3-662-53207-2. Archived from the original on 8 March 2021. Retrieved 26 March 2020.
- ↑ Siegert, Martin J.; Ellis-Evans, J. Cynan; Tranter, Martyn; Mayer, Christoph; Petit, Jean-Robert; Salamatin, Andrey; Priscu, John C. (December 2001). "Physical, chemical and biological processes in Lake Vostok and other Antarctic subglacial lakes". Nature. 414 (6864): 603–609. Bibcode:2001Natur.414..603S. doi:10.1038/414603a. PMID 11740551. S2CID 4423510.
- ↑ Davies, Bethan. "Antarctic subglacial lakes". AntarcticGlaciers. Archived from the original on 3 October 2020. Retrieved 25 March 2020.
- ↑ Masterton, William L.; Hurley, Cecile N. (2008). Chemistry: principles and reactions (6th ed.). Cengage Learning. p. 230. ISBN 978-0-495-12671-3. Archived from the original on 8 March 2021. Retrieved 3 April 2020.
- ↑ Peaco, Jim. "Yellowstone Lesson Plan: How Yellowstone Geysers Erupt". Yellowstone National Park: U.S. National Park Service. Archived from the original on 2 March 2020. Retrieved 5 April 2020.
- ↑ Brahic, Catherine. "Found: The hottest water on Earth". New Scientist. Archived from the original on 9 May 2020. Retrieved 5 April 2020.
- ↑ USDA Food Safety and Inspection Service. "High Altitude Cooking and Food Safety" (PDF). Archived from the original (PDF) on 20 January 2021. Retrieved 5 April 2020.
- ↑ "Pressure Cooking – Food Science". Exploratorium. 26 September 2019. Archived from the original on 19 June 2020. Retrieved 21 April 2020.
- ↑ Allain, Rhett (12 September 2018). "Yes, You Can Boil Water at Room Temperature. Here's How". Wired. Archived from the original on 28 September 2020. Retrieved 5 April 2020.
- ↑ "Hard Water". USGS. 8 April 2014. Retrieved 16 May 2015.
- ↑ "CFR - Code of Federal Regulations Title 21". www.accessdata.fda.gov. Retrieved 2020-12-04.
- ↑ Da Conceicao Neta, Edith Ramos; Johanningsmeier, Suzanne D.; McFeeters, Roger F. (2007). "The Chemistry and Physiology of Sour Taste—A Review". Journal of Food Science. 72 (2): R33 – R38. doi:10.1111/j.1750-3841.2007.00282.x. ISSN 1750-3841.
- ↑ "11.6: Acid-Base Reactions". Chemistry LibreTexts. 2016-05-09. Retrieved 2025-05-03.
- ↑ "Disinfection with Chlorine | Public Water Systems | Drinking Water | Healthy Water | CDC". www.cdc.gov. 10 October 2018. Retrieved 2020-04-30.
- ↑ Mirlohi, Susan (2022). "Characterization of Metallic Off-Flavors in Drinking Water: Health, Consumption, and Sensory Perception". International Journal of Environmental Research and Public Health. 19 (24): 16829. doi:10.3390/ijerph192416829. PMC 9778853. PMID 36554714.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ↑ Trivedi, Bijal P. (2012). "Gustatory system: The finer points of taste". Nature. 486 (7403): S2 – S3. Bibcode:2012Natur.486S...2T. doi:10.1038/486s2a. ISSN 0028-0836. PMID 22717400. S2CID 4325945.
- ↑ Bernadette M. Marriott, ed. (1993). "Heat as a Factor in the Perception of Taste, Smell, and Oral Sensation". Nutritional Needs in Hot Environments: Applications for Military Personnel in Field Operations. Washington, DC: National Academies Press. Retrieved 30 April 2025.
- ↑ Jüttner, Friedrich; Watson, Susan B. (July 2007). "Biochemical and Ecological Control of Geosmin and 2-methylisoborneol in Source Waters". Applied and Environmental Microbiology. 73 (14): 4395–4706. Bibcode:2007ApEnM..73.4395J. doi:10.1128/AEM.02250-06. PMC 1932821. PMID 17400777. S2CID 1954489.
- ↑ "Why Does My Water Smell Like Rotten Eggs? Hydrogen Sulfide and Sulfur Bacteria in Well Water". Minnesota Department of Health. Retrieved 2 May 2025.
- ↑ Candau, Joël (2004). "The Olfactory Experience: constants and cultural variables". Water Science and Technology. 49 (9): 11–17. Bibcode:2004WSTec..49...11C. doi:10.2166/wst.2004.0522. PMID 15237601. Archived from the original on 2 October 2016. Retrieved 28 September 2016.
- ↑ R. Llinas, W. Precht (2012), Frog Neurobiology: A Handbook. Springer Science & Business Media. ISBN 978-3642663161
- ↑ "Water Compressibility | U.S. Geological Survey". www.usgs.gov. 2019-10-22. Retrieved 2025-05-03.
- ↑ "DEFINITION OF VISCOSITY". www.princeton.edu. Retrieved 2025-05-03.
- ↑ "Speed of sound in water". oceanexplorer.noaa.gov. Retrieved 2025-05-03.
- ↑ "Calculation of absorption of sound in seawater". resource.npl.co.uk. Retrieved 2025-05-03.
- ↑ 219.0 219.1 "3.3: Electrical Properties of Pure Water". Chemistry LibreTexts. 2021-01-17. Retrieved 2025-05-03.
- ↑ 220.0 220.1 "16.3: Self-Ionization of Water and the pH Scale". Chemistry LibreTexts. 2015-01-18. Retrieved 2025-05-03.
- ↑ "Conductivity (Electrical Conductance) and Water | U.S. Geological Survey". www.usgs.gov. 2019-10-22. Retrieved 2025-05-03.
- ↑ Davis, Jim; Milligan, Mark (April 5, 2011). Why Is Bear Lake So Blue? And Other Commonly Asked Questions. Public Information Series. Vol. 96. Utah Geological Survey. p. 10. ISBN 978-1-55791-842-0. Archived from the original on 23 January 2012. Retrieved 5 October 2011.
- ↑ "Water Color | U.S. Geological Survey". www.usgs.gov. 2019-10-22. Retrieved 2025-05-03.
- ↑ "How far does light travel in the ocean? : Ocean Exploration Facts: NOAA Office of Ocean Exploration and Research". oceanexplorer.noaa.gov. Retrieved 2025-05-03.
- ↑ "Index of Refraction of Seawater and Freshwater as a Function of Wavelength and Temperature | Parrish Research Group | Oregon State University". research.engr.oregonstate.edu. Retrieved 2025-05-03.
- ↑ Valqui, Melissa (2022-08-20). "The Reaction of Sodium in Water". ChemTalk. Retrieved 2025-05-03.
- ↑ "9.4.2.1: Amphoteric nature of water". Chemistry LibreTexts. 2022-03-07. Retrieved 2025-05-03.
- ↑ 228.0 228.1 "5.4: Hydrolysis Reactions". Chemistry LibreTexts. 2021-08-04. Retrieved 2025-05-03.
- ↑ "23.9: Electrolysis of Water". Chemistry LibreTexts. 2016-06-27. Retrieved 2025-05-03.
- ↑ "What is green hydrogen and why do we need it? An expert explains". World Economic Forum. Retrieved 2025-05-03.
- ↑ "AQUASTAT Dissemination System". data.apps.fao.org. Retrieved 2025-05-04.
- ↑ 232.0 232.1 232.2 232.3 Cite error: The named reference
:6was used but no text was provided for refs named (see the help page). - ↑ Gleick, P. H., ed. (1993). Water in Crisis: A Guide to the World's Freshwater Resources. Oxford University Press. p. 15, Table 2.3. Archived from the original on 8 April 2013.
- ↑ Ben-Naim, A.; Ben-Naim, R. (2011). Alice's Adventures in Water-land. World Scientific Publishing. p. 31. doi:10.1142/8068. ISBN 978-981-4338-96-7.
- ↑ "Weathering". education.nationalgeographic.org. Retrieved 2025-05-04.
- ↑ 236.0 236.1 "5 Weathering, Erosion, and Sedimentary Rocks – An Introduction to Geology". Retrieved 2025-05-04.
- ↑ "Sediment". education.nationalgeographic.org. Retrieved 2025-05-04.
- ↑ Panchuk, Karla (2019). "7.1 Magma and How It Forms". UNIVERSITY OF SASKATCHEWAN.
- ↑ "ALMA greatly improves capacity to search for water in universe". Archived from the original on 23 July 2015. Retrieved 20 July 2015.
- ↑ "Ocean Worlds". Ocean Worlds. Retrieved 2025-05-05.
- ↑ Clavin, Whitney; Buis, Alan (22 July 2011). "Astronomers find largest, most distant reservoir of water". NASA. Archived from the original on 24 July 2011. Retrieved 25 July 2011.
- ↑ Staff (22 July 2011). "Astronomers find largest, oldest mass of water in Universe". Space.com. Archived from the original on 29 October 2011. Retrieved 23 July 2011.
- ↑ "NASA's Hubble Finds Water Vapor in Small Exoplanet's Atmosphere - NASA Science". 2024-01-25. Retrieved 2025-05-05.
- ↑ "Hubble Finds Evidence of Persistent Water Vapor in One Hemisphere of Europa - NASA Science". 2021-10-14. Retrieved 2025-05-05.
- ↑ "Discovery Alert: Webb Maps and Finds Traces of Water in an Ultra-hot Gas Giant's Atmosphere - NASA Science". 2023-05-31. Retrieved 2025-05-05.
- ↑ "Herschel detects abundant water in planet-forming disc". www.esa.int. Retrieved 2025-05-05.
- ↑ "Water in the early Universe". www.mpg.de. Retrieved 2025-05-05.
- ↑ "Enceladus - NASA Science". 2018-08-21. Retrieved 2025-05-05.
- ↑ "Galileo Evidence Points to Possible Water World Under Europa's Icy Crust - NASA Science". 2000-08-25. Retrieved 2025-05-05.
- ↑ "NASA Confirms Evidence That Liquid Water Flows on Today's Mars - NASA". Retrieved 2025-05-05.
- ↑ "Global map of hydrated minerals on Mars". www.esa.int. Retrieved 2025-05-05.
- ↑ "The Solar System and Beyond is Awash in Water". NASA Jet Propulsion Laboratory (JPL). Retrieved 2025-05-06.
- ↑ "Water Found on Sun". solar-center.stanford.edu. Retrieved 2025-05-06.
- ↑ "Ice on the Moon". nssdc.gsfc.nasa.gov. Retrieved 2025-05-06.
- ↑ "Water ice on Mercury". nssdc.gsfc.nasa.gov.
- ↑ "NASA's LRO Sheds Light on Lunar Water Movement - NASA". 2019-03-08. Retrieved 2025-05-06.
- ↑ "MESSENGER - NASA Science". 2017-12-20. Retrieved 2025-05-06.
- ↑ "Where did Venus's water go?". www.esa.int. Retrieved 2025-05-06.
- ↑ "Ceres: Facts - NASA Science". 2017-11-09. Retrieved 2025-05-06.
- ↑ "NASA's Bennu Asteroid Sample Contains Carbon, Water - NASA". Retrieved 2025-05-06.
- ↑ "SwRI scientists identify water molecules on asteroids for the first time | Southwest Research Institute". www.swri.org. Retrieved 2025-05-06.
- ↑ "Comets". 2017-10-26. Retrieved 2025-05-06.
- ↑ "Oort Cloud". 2023-06-20. Retrieved 2025-05-06.
- ↑ "Findings From NASA's Juno Update Jupiter Water Mystery - NASA". 2020-02-18. Retrieved 2025-05-06.
- ↑ Institute, Planetary Science. "The water in Saturn's rings and satellites is like that on Earth except for moon Phoebe, which is out of this world". phys.org. Retrieved 2025-05-06.
- ↑ "Uranus: Facts - NASA Science". 2017-11-10. Retrieved 2025-05-06.
- ↑ "Neptune: Facts - NASA Science". 2017-11-10. Retrieved 2025-05-06.
- ↑ "Neptune: Facts - NASA Science". 2017-11-10. Retrieved 2025-05-06.
- ↑ "Pluto Has an Ocean of Liquid Water Surrounded by a 40-80 km Ice Shell". Universe Today. Retrieved 2025-05-06.
- ↑ Oliver, Amy C.; Observatory, National Radio Astronomy. "Tracing the history of water in planet formation back to the interstellar medium". phys.org. Retrieved 2025-05-06.
- ↑ Dulieu, F.; Amiaud, L.; Congiu, E.; Fillion, J.-H.; Matar, E.; Momeni, A.; Pirronello, V.; Lemaire, J. L. (2010-03-01). "Experimental evidence for water formation on interstellar dust grains by hydrogen and oxygen atoms". Astronomy & Astrophysics. 512: A30. doi:10.1051/0004-6361/200912079. ISSN 0004-6361.
- ↑ Vidali, Gianfranco; Jing, Dapeng; He, Jiao (2012). "Formation of water in the interstellar medium". 39th COSPAR Scientific Assembly. 39: 2086.
- ↑ Melnick, Gary J.; Tolls, Volker; Snell, Ronald L.; Kaufman, Michael J.; Bergin, Edwin A.; Goicoechea, Javier R.; Goldsmith, Paul F.; González-Alfonso, Eduardo; Hollenbach, David J.; Lis, Dariusz C.; Neufeld, David A. (2020). "Distribution of Water Vapor in Molecular Clouds. II". The Astrophysical Journal. 892 (1): 22. doi:10.3847/1538-4357/ab77b4. ISSN 0004-637X.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ↑ Melnick, G. J.; Bergin, E. A. (2005-01-01). "The legacy of SWAS: Water and molecular oxygen in the interstellar medium". Advances in Space Research. Infrared/Submm Astronomy from Space. 36 (6): 1027–1030. doi:10.1016/j.asr.2005.05.110. ISSN 0273-1177.
- ↑ "A Planetary Disk in the Orion Nebula is Destroying and Replenishing Oceans of Water Every Month". Universe Today. Retrieved 2025-05-06.
- ↑ "ESA Science & Technology - Water in Orion". sci.esa.int. Retrieved 2025-05-06.
- ↑ Guélin, Michel; Cernicharo, Jose (2022). "Organic Molecules in Interstellar Space: Latest Advances". Frontiers in Astronomy and Space Sciences. 9. doi:10.3389/fspas.2022.787567. ISSN 2296-987X.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ↑ SpaceRef (2023-07-12). "Webb's Stunning View Of Rho Ophiuchi". SpaceNews. Retrieved 2025-05-06.
- ↑ "Young Stars in Their Baby Blanket of Dust: Rho Ophiuchi". www.spitzer.caltech.edu. Retrieved 2025-05-06.
- ↑ "Zoom in to Rho Ophiuchi". Webb. Retrieved 2025-05-06.
- ↑ "ALMA Traces History of Water in Planet Formation Back to the Interstellar Medium | ALMA Observatory". Retrieved 2025-05-06.
- ↑ "How We Find and Characterize - NASA Science". 2020-10-26. Retrieved 2025-05-31.
- ↑ "Water, water everywhere - on an extrasolar planet - NASA Science". 2007-11-07. Retrieved 2025-05-31.
- ↑ Sánchez-López, A.; Alonso-Floriano, F. J.; López-Puertas, M.; Snellen, I. a. G.; Funke, B.; Nagel, E.; Bauer, F. F.; Amado, P. J.; Caballero, J. A.; Czesla, S.; Nortmann, L. (2019-10-01). "Water vapor detection in the transmission spectra of HD 209458 b with the CARMENES NIR channel". Astronomy & Astrophysics. 630: A53. doi:10.1051/0004-6361/201936084. ISSN 0004-6361.
- ↑ "Webb Discovers Methane, Carbon Dioxide in Atmosphere of K2-18 b - NASA". 2023-09-11. Retrieved 2025-05-31.
- ↑ "NASA Astrobiology". astrobiology.nasa.gov. Retrieved 2025-05-31.
- ↑ Anderson, Paul Scott (2021-03-23). "Subsurface oceans on alien worlds even better for life than Earth's oceans? | Space | EarthSky". earthsky.org. Retrieved 2025-05-31.
- ↑ "Ingredients for Life | Why Europa". NASA's Europa Clipper. Retrieved 2025-05-31.
- ↑ "Enceladus - NASA Science". 2018-08-21. Retrieved 2025-05-31.
- ↑ "Signs of ancient flowing water on Mars". www.esa.int. Retrieved 2025-05-31.
- ↑ "NASA's Curiosity Searches for New Clues About Mars' Ancient Water - NASA". 2024-03-29. Retrieved 2025-05-31.
- ↑ "NASA Confirms Evidence That Liquid Water Flows on Today's Mars - NASA". Retrieved 2025-05-31.
- ↑ "The Habitable Zone - NASA Science". 2020-10-26. Retrieved 2025-05-31.
- ↑ "NASA's Hubble Finds Water Vapor in Small Exoplanet's Atmosphere - NASA Science". 2024-01-25. Retrieved 2025-05-31.
- ↑ "Percentage of water". Archived from the original on 2013-12-14. Retrieved 2008-12-11.
- ↑ "Fresh water percentage (2)". Archived from the original on 2007-07-15. Retrieved 2008-12-11.
- ↑ "Water Law Overview – National Agricultural Law Center". Retrieved 2025-06-03.
- ↑ "Land and water - the rights interface". www.fao.org. Retrieved 2025-06-03.
- ↑ "riparian doctrine". LII / Legal Information Institute. Retrieved 2025-06-03.
- ↑ "Western Water Law: Understanding the Doctrine of Prior Appropriation". Extension | University of Nevada, Reno. Retrieved 2025-06-03.
- ↑ Ramazzott, Marco. Customary Water Rights and Contemporary Water Legislation (PDF). Fao Legal Papers Online.
- ↑ Masoumeh, Zeinali; Bozorg-Haddad, Omid; Singh, Vijay P. (2021-01-01), Bozorg-Haddad, Omid (ed.), "6 - Rights and international laws of transboundary water resources", Economical, Political, and Social Issues in Water Resources, Elsevier, pp. 103–129, doi:10.1016/b978-0-323-90567-1.00013-9, ISBN 978-0-323-90567-1, retrieved 2025-06-03
- ↑ ". Global Affairs and Strategic Studies. Facultad de Derecho". Global Affairs and Strategic Studies (in European Spanish). Retrieved 2025-06-03.
- ↑ "Water Resources Management". World Bank. Retrieved 2025-06-02.
- ↑ US EPA, OP (2013-02-22). "Summary of the Clean Water Act". www.epa.gov. Retrieved 2025-06-02.
- ↑ "Water dispute on the Nile River could destabilize the region". USC Today. 2021-07-13. Retrieved 2025-06-02.
- ↑ "Human Rights to Water and Sanitation". UN-Water. Retrieved 2025-06-02.
- ↑ "Water Scarcity". UN-Water. Retrieved 2025-06-03.
- ↑ Fuller, Amy C.; Harhay, Michael O. (2010-06-30). "Population Growth, Climate Change and Water Scarcity in the Southwestern United States". American Journal of Environmental Sciences. 6 (3): 249–252. doi:10.3844/ajessp.2010.249.252. ISSN 1553-345X. PMC 3071514. PMID 21479150.
- ↑ Vega, Edinson Cueva; Dávila, Manuel Antonio Morante; Castillo, Percy Zuta; Espinoza, Oscar Chavez; Barrientos, Marden Rigoberto Zumaeta (2023-11-14). "Population Growth and Water Consumption: Chachapoyas Case, 2011 - 2021". Journal of Law and Sustainable Development. 11 (11): e1156. doi:10.55908/sdgs.v11i11.1156. ISSN 2764-4170.
- ↑ He, Chunyang; Liu, Zhifeng; Wu, Jianguo; Pan, Xinhao; Fang, Zihang; Li, Jingwei; Bryan, Brett A. (2021-08-03). "Future global urban water scarcity and potential solutions". Nature Communications. 12 (1): 4667. doi:10.1038/s41467-021-25026-3. ISSN 2041-1723. PMC 8333427. PMID 34344898.
- ↑ "Water Pollution Definition - Types, Causes, Effects". www.nrdc.org. 2023-01-11. Retrieved 2025-06-04.
- ↑ Lin, Li; Yang, Haoran; Xu, Xiaocang (2022-06-30). "Effects of Water Pollution on Human Health and Disease Heterogeneity: A Review". Frontiers in Environmental Science. 10. doi:10.3389/fenvs.2022.880246. ISSN 2296-665X.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ↑ Fava, M. Fava (2022-05-09). "Plastic pollution in the ocean: data, facts, consequences". Ocean Literacy Portal. Retrieved 2025-06-04.
- ↑ Lee, Yongjin; Cho, Jaelim; Sohn, Jungwoo; Kim, Changsoo (2023). "Health Effects of Microplastic Exposures: Current Issues and Perspectives in South Korea". Yonsei Medical Journal. 64 (5): 301–308. doi:10.3349/ymj.2023.0048. ISSN 1976-2437. PMC 10151227. PMID 37114632.
- ↑ "Crops and Drops". www.fao.org. Retrieved 2025-06-04.
- ↑ Ritchie, Hannah; Roser, Max (2018-07-01). "Water Use and Stress". Our World in Data.
- ↑ Abraham, Martin A., ed. (2017). Encyclopedia of sustainable technologies. Amsterdam, The Netherlands: Elsevier. ISBN 978-0-12-804792-7.
- ↑ Bruenig, E (2002). "The concept of estimating the risk of water losses in the water supply network". Journal of Environmental Management. 66 (3): 341–343. doi:10.1016/s0301-4797(02)90581-5. ISSN 0301-4797.
- ↑ "Religion and water". University of Bergen. Retrieved 2025-05-28.
- ↑ @aithor (2024-06-28). "The Symbolism of Water in Various Religious Traditions". aithor.com. Retrieved 2025-05-28.
- ↑ "Baptism | Meaning, Rituals & History | Britannica". www.britannica.com. 2025-05-17. Retrieved 2025-05-28.
- ↑ "Method and Rulings of Wudhu'". al-islam.org. 2016-03-15. Retrieved 2025-05-28.
- ↑ "Ghusl | Ritual Cleansing, Ablution & Purification | Britannica". www.britannica.com. Retrieved 2025-05-28.
- ↑ Mitra, Piyali (2023), Basu, Mrittika; DasGupta, Rajarshi (eds.), "Water Symbolism in Hindu Culture", Indigenous and Local Water Knowledge, Values and Practices, Singapore: Springer Nature, pp. 39–46, doi:10.1007/978-981-19-9406-7_3, ISBN 978-981-19-9406-7, retrieved 2025-05-28
- ↑ 326.0 326.1 "Why is the Ganges River considered holy in Hinduism? | Britannica". www.britannica.com. Retrieved 2025-05-28.
- ↑ "Buddhist Perspective on Water: Insights and Reflections – IILSS-International institute for Law of the Sea Studies". Retrieved 2025-05-28.
- ↑ "Mikvah | Ritual Bath, Immersion, Purification | Britannica". www.britannica.com. 2025-05-15. Retrieved 2025-05-28.
- ↑ "Sikh pilgrims perform ritual baths as Vaisakhi festival kicks off in Pakistan". Arab News. 2019-04-13. Retrieved 2025-05-28.
- ↑ Todd, Wendy F. K’ah Skáahluwáa; Northbird, Arianna V.; Towne, Chessaly E. (2023-05-23). "The Science in Indigenous Water Stories, Indigenous Women's Connection to Water". Open Rivers Journal. Retrieved 2025-05-28.
- ↑ "Pilgrimage | Meaning, Examples, Religions, Places, & Sites | Britannica". www.britannica.com. Retrieved 2025-05-28.
- ↑ "Lourdes - Special places - GCSE Religious Studies Revision - WJEC". BBC Bitesize. Retrieved 2025-05-28.
- ↑ "1. Miracles and Religion", Islam, Christianity and the Realms of the Miraculous, Edinburgh University Press, pp. 1–26, 2018-11-16, doi:10.1515/9780748699070-003, ISBN 978-0-7486-9907-0, retrieved 2025-05-28
- ↑ "Aristotle". web.sbu.edu. Retrieved 2025-05-28.
- ↑ Gordon, Ronald D. "Ancient Chinese Philosophy and the Wisdom of Water" (PDF). Hawaii Journal of the Humanities. 4(1). ISSN 2694-6165.
- ↑ Hansen, Chad (2025), Zalta, Edward N.; Nodelman, Uri (eds.), "Daoism", The Stanford Encyclopedia of Philosophy (Summer 2025 ed.), Metaphysics Research Lab, Stanford University, retrieved 2025-05-28
- ↑ www.wisdomlib.org (2015-07-14). "Pancabhuta, Pañcabhūta, Panca-bhuta, Pancan-bhuta, Pamcabhuta: 19 definitions". www.wisdomlib.org. Retrieved 2025-05-28.
- ↑ Wiley (2005-09-09). Encyclopedia of Life Sciences (1 ed.). Wiley. doi:10.1002/9780470015902.a0003607.pub3. ISBN 978-0-470-01617-6.
- ↑ "Phenomenology - Water: experiencing movement". tomvangelder.antrovista.com. Retrieved 2025-05-28.
- ↑ "River Gods, Lake Monsters, and the Abiding Power of Myth". Science History Institute. Retrieved 2025-05-30.
- ↑ "Kappa | Yokai.com". yokai.com. Retrieved 2025-05-30.
- ↑ Briggs, Katharine Mary (1979). A dictionary of fairies: hobgoblins, brownies, bogies, and other supernatural creatures. Penguin book (Reprint ed.). Harmondsworth: Penguin Books. ISBN 978-0-14-004753-0.
- ↑ 343.0 343.1 "Mami Wata (African myth) | EBSCO Research Starters". www.ebsco.com. Retrieved 2025-05-30.
- ↑ "Siren (mythology) | EBSCO Research Starters". www.ebsco.com. Retrieved 2025-05-30.
- ↑ O'Brien, Renee (2017-04-29). "The Final Journey: Crossing the Styx". Hellenic Museum. Retrieved 2025-05-30.
- ↑ "The Celts: the people "at the end of the world" | Columbia Theological Seminary | Atlanta, GA". Columbia Theological Seminary. 2017-05-11. Archived from the original on 2019-11-05. Retrieved 2025-05-30.
- ↑ MacKillop, James (2000). A dictionary of Celtic mythology. Oxford paperback reference. Oxford: Oxford University Press. ISBN 978-0-19-280120-3.
- ↑ "Japan - Death Folklore & Doll Marriage". Myend. Retrieved 2025-05-30.
- ↑ LDJ_ZEN (2023-07-24). "Shamanic Significance of Water - Born As The Earth (BEZA)". Retrieved 2025-05-30.
- ↑ Mark, Joshua J. "Enuma Elish - The Babylonian Epic of Creation - Full Text". World History Encyclopedia. Retrieved 2025-05-30.
- ↑ Denova, Rebecca. "Noah's Ark". World History Encyclopedia. Retrieved 2025-05-30.
- ↑ "Creation stories in Hinduism - The existence of God - GCSE Religious Studies Revision - CCEA". BBC Bitesize. Retrieved 2025-05-30.
- ↑ Mark, Joshua J. "Ragnarök". World History Encyclopedia. Retrieved 2025-05-30.
- ↑ Cusack, Carole M.; Wilson, Dominique Beth (2016-10-03). "Scotland's Sacred Waters: Holy Wells and Healing Springs". Journal of the Sydney Society for Scottish History. 16. ISSN 1320-4246.
- ↑ Aldhouse-Green, Miranda Jane (1993). The gods of the Celts. Dover (N.H.): A. Sutton. ISBN 978-0-86299-292-7.
- ↑ "CATHOLIC ENCYCLOPEDIA: Lourdes". www.newadvent.org. Retrieved 2025-05-30.
- ↑ "Japanese Religion Through the Lens of Water". Kyoto Journal. 2022-04-12. Retrieved 2025-05-30.
- ↑ "Fountain of Youth | EBSCO Research Starters". www.ebsco.com. Retrieved 2025-05-30.
- ↑ "Lethe | River of Forgetfulness, Underworld River, Mythical River | Britannica". www.britannica.com. Retrieved 2025-05-30.
- ↑ "Water as Science and Art". Magazine. Retrieved 2025-06-01.
- ↑ "Water". Google Arts & Culture. Retrieved 2025-06-01.
- ↑ "Piero della Francesca | The Baptism of Christ | NG665 | National Gallery, London". www.nationalgallery.org.uk. Retrieved 2025-06-01.
- ↑ "Smarthistory – The Great Relief at Mamallapuram". smarthistory.org. Retrieved 2025-06-01.
- ↑ "Divine Currents : the Ganga and Yamuna in Indian Art - Enroute Indian History Divine Currents : the Ganga and Yamuna in Indian Art". Enroute Indian History. 2024-08-21. Retrieved 2025-06-01.
- ↑ "Poseidon: Tamer of horses, Earth-Shaker & God of the sea - (Phillip TIjerina)". Google Arts & Culture. Retrieved 2025-06-01.
- ↑ Hays, Jeffrey. "Art in Ancient Egypt: Themes, Ideas, Materials, Subjects, Styles, Influences | Middle East And North Africa — Facts and Details". africame.factsanddetails.com. Retrieved 2025-06-01.
- ↑ "Hokusai Under the Wave off Kanagawa | U.S. Geological Survey". www.usgs.gov. Retrieved 2025-06-01.
- ↑ Monet, Claude (1906), Water Lilies, retrieved 2025-06-01
- ↑ "Claude Monet. Water Lilies. 1914-26 | MoMA". The Museum of Modern Art. Retrieved 2025-06-01.
- ↑ "Watercolor". The Metropolitan Museum of Art. Retrieved 2025-06-01.
- ↑ "Crown Fountain". Millennium Park Foundation. Retrieved 2025-06-01.
- ↑ "Rain Room | Sharjah Art Foundation". www.sharjahart.org. Retrieved 2025-06-01.
- ↑ Ryan, Brendan. "LibGuides: English: Water in Literature". clonard.libguides.com. Retrieved 2025-06-01.
- ↑ "Water Symbolism - Meanings in Literature and Culture - Literary Devices". 2023-05-22. Retrieved 2025-06-01.
- ↑ "The Old Man and the Sea | Summary, Characters, & Facts | Britannica". www.britannica.com. Retrieved 2025-06-01.
- ↑ "Moby Dick | Summary, Characters, Author, Importance, & Facts | Britannica". www.britannica.com. 2025-04-23. Retrieved 2025-06-01.
- ↑ "Odyssey | Summary, Characters, Meaning, & Facts | Britannica". www.britannica.com. 2025-04-19. Retrieved 2025-06-01.
- ↑ "The Secret Garden | Summary, Characters, & Facts | Britannica". www.britannica.com. 2025-05-17. Retrieved 2025-06-01.
- ↑ "Adventures of Huckleberry Finn: Analysis of Setting | EBSCO Research Starters". www.ebsco.com. Retrieved 2025-06-01.
- ↑ "The Rime of the Ancient Mariner: Analysis of Setting | EBSCO Research Starters". www.ebsco.com. Retrieved 2025-06-01.
- ↑ "Orpheus and Eurydice: Analysis of Setting | EBSCO Research Starters". www.ebsco.com. Retrieved 2025-06-01.
- ↑ "Analysing water and it's significance in film –". www.mediafactory.org.au. Retrieved 2025-06-01.
- ↑ "Titanic | Movie, Characters, Summary, Cast, & Facts | Britannica". www.britannica.com. 2025-05-01. Retrieved 2025-06-01.
- ↑ "Avatar | Film Series, Plot, Reception, Colonialism, & Facts | Britannica". www.britannica.com. Retrieved 2025-06-01.
- ↑ "Jaws | Shark, Steven Spielberg, Blockbuster, & Facts | Britannica". www.britannica.com. 2025-05-26. Retrieved 2025-06-01.
- ↑ 386.0 386.1 386.2 "'Dihydrogen monoxide' hoax returns as social media meme | AAP". aap.com.au. Retrieved 2025-06-02.
- ↑ "Dihydrogen Monoxide Research Division - dihydrogen monoxide info". dhmo.org. Retrieved 2025-06-02.
- ↑ "Water in Music | NLS Music Notes". Library of congress.
- ↑ "La Mer". BSO. Retrieved 2025-06-02.
- ↑ "The elements of music to consider - Chopin: Prelude, Op.28, No.15 - AQA - GCSE Music Revision - AQA". BBC Bitesize. Retrieved 2025-06-02.
- ↑ "Bridge Over Troubled Water". The Paul Simon Official Site. Retrieved 2025-06-02.
- ↑ "The Powerful Meaning of Joni Mitchell's Sad Christmas Song, "River"". Benedictine College Media & Culture. 2023-12-19. Retrieved 2025-06-02.
- ↑ "Rain dance | anthropology | Britannica". www.britannica.com. Retrieved 2025-06-02.
- ↑ Talkeu Tounouga, Camille; Brock, Odile (2003). "The Symbolic Function of Water in Sub-Saharan Africa: A Cultural Approach". Leonardo. 36 (4): 283–283. ISSN 1530-9282.
- ↑ "MS River Blues". The Mississippi Blues Trail. Retrieved 2025-06-02.
- ↑ Montagne, Vicky Mogollón. "Women and Water: Connections in Caribbean Music and Spirituality". Smithsonian Folklife Festival. Retrieved 2025-06-02.
- ↑ "Dance To The Beat Of The Origins And Other Facts About 12 Caribbean Music Styles". Dictionary.com. 2022-03-03. Retrieved 2025-06-02.
- ↑ MusicalFlora (2025-01-16). "The Role of Nature Sounds in Ambient Music: A Complete Guide". Musical Flora. Retrieved 2025-06-02.
- ↑ Kwon, Chan-Young; Kim, Hyunsu; Kim, Sung-Hee (2024-02-05). "The Modernization of Oriental Music Therapy: Five-Element Music Therapy Combined with Artificial Intelligence". Healthcare (Basel, Switzerland). 12 (3): 411. doi:10.3390/healthcare12030411. ISSN 2227-9032. PMC 10855257. PMID 38338296.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
Other websites
[change | change source]- Importance of Water Archived 2021-09-28 at the Wayback Machine





